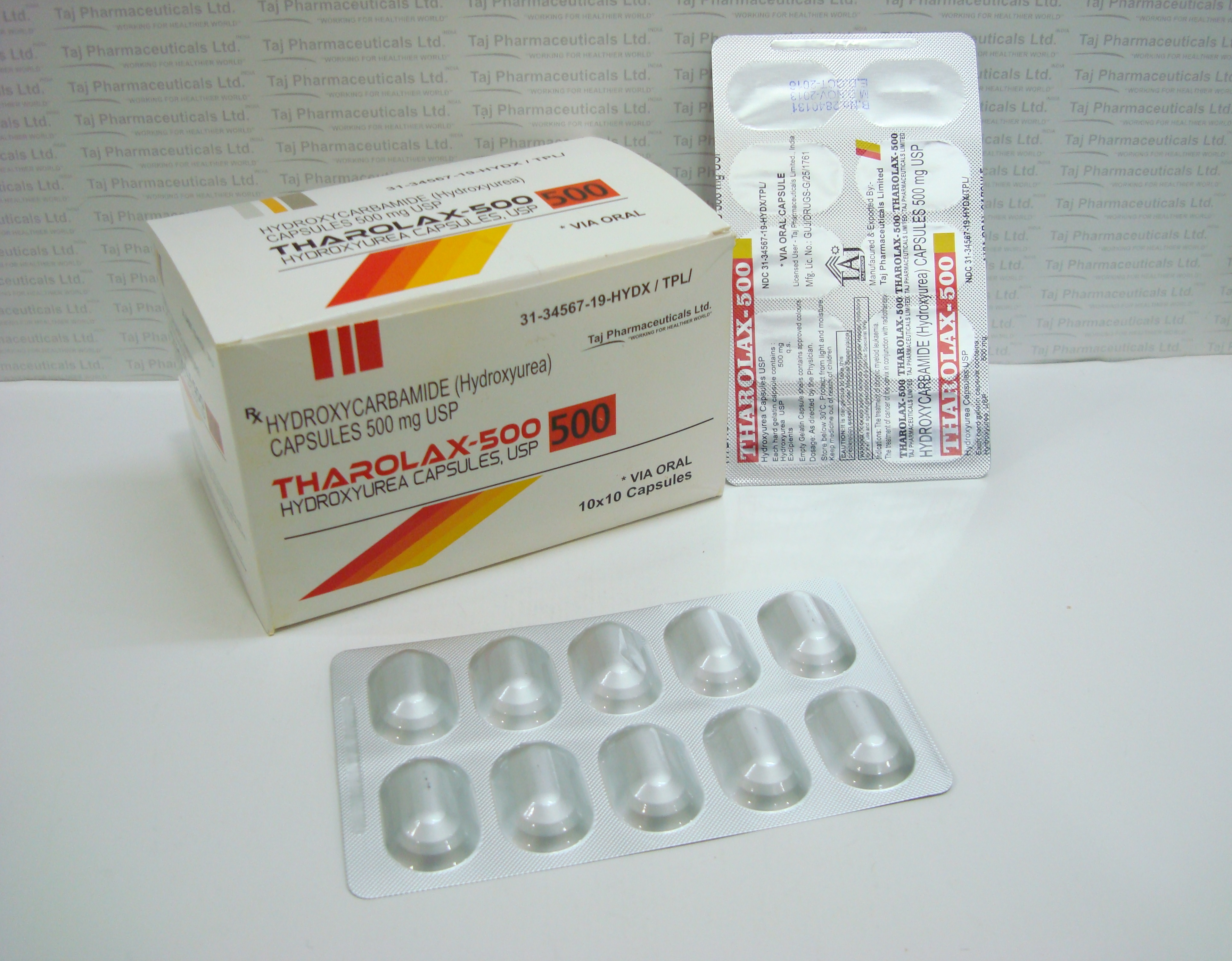
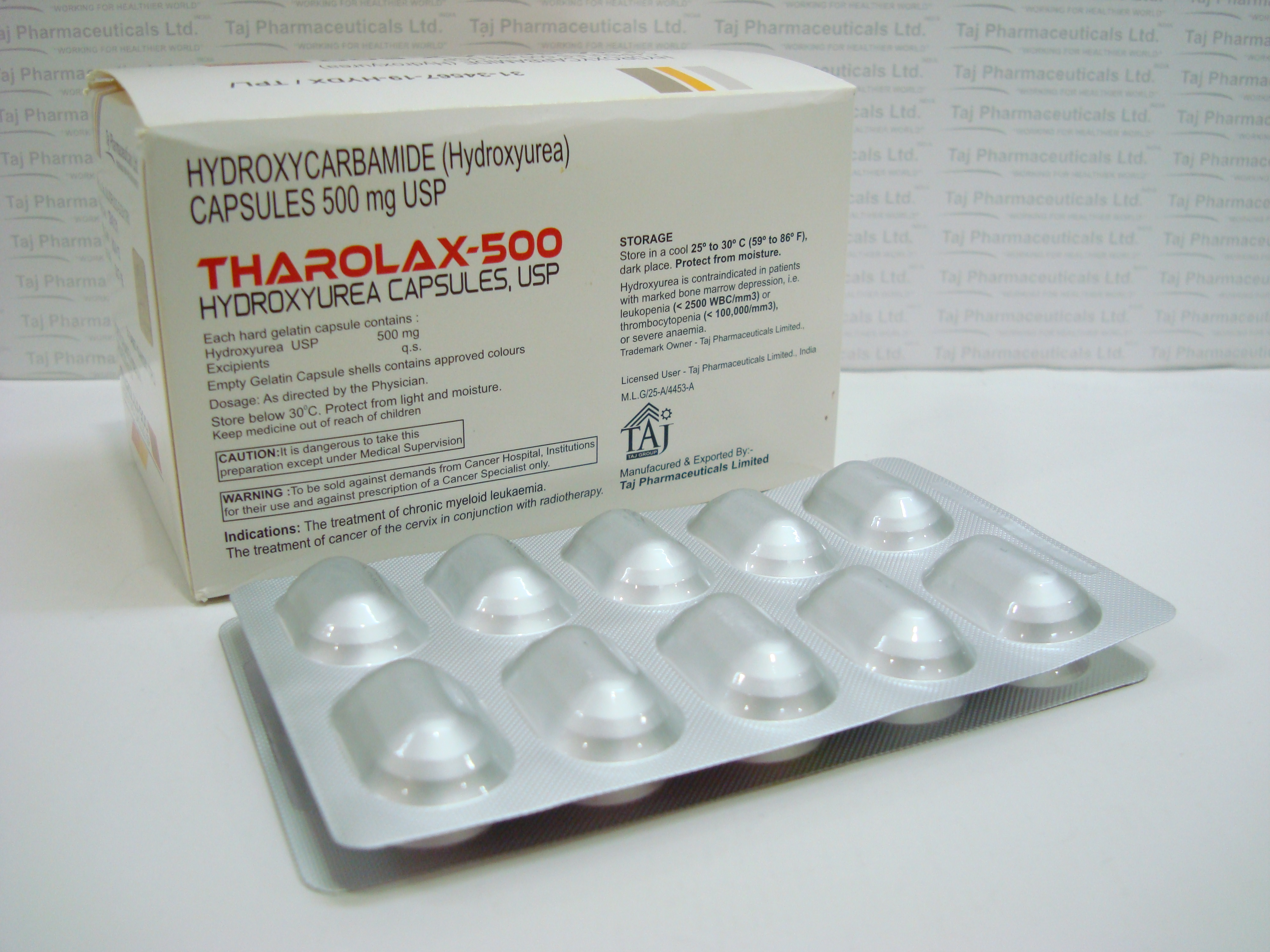
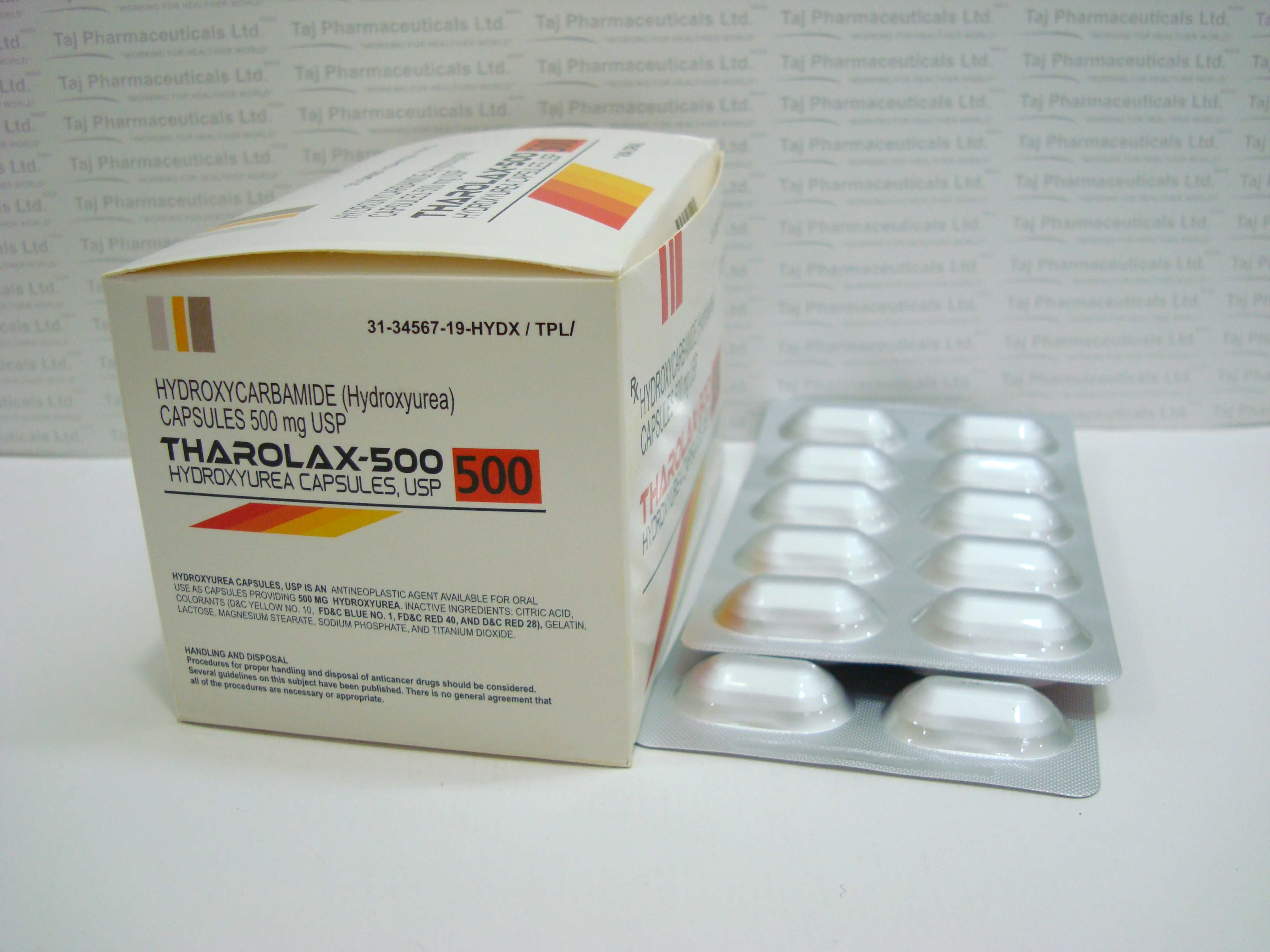
(Hydroxyurea) 500mg Capsule
Hydroxyurea is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults with sickle cell anemia. How Hydroxyurea works is not certain but it may work by reducing the number of white blood cells and/or increasing red blood cells that carry fetal hemoglobin (HbF). Fetal hemoglobin may prevent sickling.
Hydroxyurea is used to treat various types of cancers such as melanoma, leukemia and cancer of the ovary. Hydroxyurea contains hydroxyurea, an anti-cancer medicine. It interferes with the replication of cells and causes cell death, particularly in cancer cells.
Ingredients
Active:
The active ingredient in Tharolax capsules is hydroxyurea.
Each capsule contains 500mg of hydroxyurea.
Inactive:
Each capsule also contains citric acid, lactose, magnesium stearate, sodium phosphate & capsule colourants.
Storage
Hydroxyurea capsules should be stored below 30°C in a cool dry place. The container must be tightly closed.
Do not store Hydroxyurea or any other medicine in the bathroom or near a sink.
Do not leave it in the car on hot days. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-anda- half metres above the ground is a good place to store medicines. Pay particular attention to this when you are opening the capsules to dissolve the contents in water.
Disposal
If your doctor tells you to stop taking Hydroxyurea, return any that you have not taken to your treating clinic/ hospital, doctor or pharmacist, so they can be disposed of safely.
Rx only
Tharolax® (hydroxyurea capsules, USP)
500 mg per capsule
What is hydroxyurea Hydroxyurea?
Hydroxyurea is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults with sickle cell anemia. How Hydroxyurea works is not certain but it may work by reducing the number of white blood cells and/or increasing red blood cells that carry fetal hemoglobin (HbF). Fetal hemoglobin may prevent sickling.
Who should not take Hydroxyurea capsules?
Do not take Hydroxyurea capsules if you are allergic to any of the ingredients. Besides the active ingredient hydroxyurea, Hydroxyurea capsules contain the following inactive ingredients: citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colorants. Tell your doctor if you think you have ever had an allergic reaction.
If you get pregnant, Hydroxyurea may harm or cause death to your unborn child. You should not become pregnant while taking Hydroxyurea. Make sure you use a contraceptive method. Tell your doctor if you become pregnant or plan to become pregnant while taking Hydroxyurea.
How do I take Hydroxyurea capsules?
Always follow your doctor’s instructions carefully when taking Hydroxyurea capsules or any prescription medication. The usual dose of Hydroxyurea may range from as few as one to several capsules per day. Hydroxyurea is usually taken once a day. You should try to take it at the same time each day. Your doctor will determine the proper starting dose of Hydroxyurea for you based on your weight and blood count. The dose will then be increased slowly to your maximum tolerated dose (maximum dose that does NOT produce severely low blood counts). Your doctor should measure your blood counts every two weeks after you begin treatment with Hydroxyurea. Depending on the results, your dosage may be adjusted or the drug may be stopped for a while.
If you accidentally take an overdose of Hydroxyurea capsules, seek medical attention immediately. Contact your doctor, local Poison Control Center, or emergency room.
How do I handle Hydroxyurea capsules safely?
Hydroxyurea is a medication that must be handled with care. People who are not taking Hydroxyurea should not be exposed to it. To decrease the risk of exposure, wear disposable gloves when handling Hydroxyurea or bottles containing Hydroxyurea. Anyone handling Hydroxyurea should wash their hands before and after contact with the bottle or capsules. If the powder from the capsule is spilled, it should be wiped up immediately with a damp disposable towel and discarded in a closed container, such as a plastic bag. Hydroxyurea should be kept out of the reach of children and pets. Contact your doctor or pharmacist for instructions on how to dispose of outdated capsules.
What if I miss a dose of Hydroxyurea capsules?
Try not to miss your dose of Hydroxyurea, but if you do, take it as soon as possible. If it is almost time for your next dose, skip the missed dose and resume your regular dosing schedule. Do not take two doses during the same day. If you miss more than one dose, call your doctor for instructions.
What should I avoid while taking Hydroxyurea capsules?
Some other medications can increase your risk of experiencing serious side effects from Hydroxyurea. While you are taking Hydroxyurea capsules, you should inform your doctor of all prescription and over-the-counter medicines that you are taking.
In nursing mothers, Hydroxyurea is present in breast milk. Because of the potential for side effects in the newborn, you should discontinue nursing your baby while taking Hydroxyurea.
What are the possible side effects of Hydroxyurea capsules?
As with other medicines, Hydroxyurea may cause unwanted effects, although it is not always possible to tell whether such effects are caused by Hydroxyurea, another medication you may be taking, or your sickle cell anemia. Any side effects or unusual symptoms that you experience should be reported to your doctor, particularly if they persist or are troublesome.
The most serious side effects of Hydroxyurea involve the blood, and may include severely low white blood cell counts (leukopenia, neutropenia), which can decrease your resistance to infections, severely low red blood cell counts (anemia), or severely low platelet counts (thrombocytopenia), which can cause bleeding. Almost all patients who received Hydroxyurea in clinical studies needed to have their medication stopped for a time to allow their low blood counts to return to acceptable levels.
The side effects reported most often by adults with sickle cell anemia participating in studies of Hydroxyurea included hair loss, skin rash, fever, stomach and/or bowel disturbances, weight gain, bleeding, virus infection, and discolored nails (melanonychia), but these were equally common in people getting a placebo (sugar pill).
Skin cancer and leukemia, which can be fatal, have been reported in patients receiving long-term hydroxyurea for conditions other than sickle cell anemia. In laboratory tests, Hydroxyurea causes changes in chromosomes and DNA (genetic material) that strongly suggest it can cause cancer in people, especially if it is taken for a long time.
Skin ulcers have been seen in patients taking Hydroxyurea therapy. Contact your doctor if skin ulcers develop while you are taking Hydroxyurea.
Are regular blood counts necessary while taking Hydroxyurea capsules?
Yes. Your doctor should measure your blood counts every two weeks while you are taking Hydroxyurea. Your Hydroxyurea dose will require adjustment based on these regular blood counts. Serious problems can occur if the Hydroxyurea dose is not adjusted on time.What else should I know about Hydroxyurea capsules?
If you have kidney or liver disease, close monitoring of your blood count, kidney and liver function will be required. If you have kidney disease, your dose of Hydroxyurea may be started at a lower level and increased gradually.
Because it may not be possible to detect a deficiency of folic acid in patients taking Hydroxyurea, your doctor may prescribe a folic acid supplement for you.What else should I do to control my sickle cell crises?
Because painful crises can be brought on by factors such as infection, dehydration, worsening anemia, emotional stress, extreme temperature exposure, or ingestion of substances such as alcohol or other recreational drugs, you should be aware of the following general guidelines that will help keep you pain-free:
- Seek immediate medical attention when a fever develops or signs of infection appear.
- Avoid smoking and drinking more than 1 to 2 alcoholic beverages a day.
- Drink 8 to 10 glasses of water or other fluid each day.
- Avoid any types of physical exertion that seem to bring on painful crises or other discomfort.
- Avoid extreme temperature changes and dress appropriately in hot and cold weather.
What should I know if I am HIV-positive?
Because of serious, life-threatening side effects associated with Hydroxyurea used in combination with certain medications for HIV, your doctor should closely monitor your pancreas and liver function with frequent physical examinations and laboratory blood tests. The combination of Hydroxyurea, stavudine and didanosine should be avoided. Some studies have shown a decrease in the number of CD4 (T-cells) for HIV-positive patients taking Hydroxyurea. Although Hydroxyurea is approved by the U.S. Food and Drug Administration for treating sickle cell anemia, it is not approved for treating HIV infection.
This medicine was prescribed for your particular condition. Do not use Hydroxyurea capsules for another condition or give it to others. This summary does not include everything there is to know about Hydroxyurea capsules. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you have questions or concerns, or want more information about Hydroxyurea capsules, your physician and pharmacist have the complete prescribing information upon which this guide is based. You may want to read it and discuss it with your doctor. Remember, no written summary can replace careful discussion with your doctor.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufacturer
Hydroxyurea ® capsules is made in India by:
Taj Pharmaceuticals Limited.Hydroxyurea is a trademark of a Taj Pharma Company
Rx only
Tharolax® (hydroxyurea capsules, USP)
500 mg per capsule
WARNING
Treatment of patients with Hydroxyurea may be complicated by severe, sometimes life-threatening, adverse effects. Hydroxyurea should be administered under the supervision of a physician experienced in the use of this medication for the treatment of sickle cell anemia.
Hydroxyurea is mutagenic and clastogenic, and causes cellular transformation to a tumorigenic phenotype. Hydroxyurea is thus unequivocally genotoxic and a presumed transspecies carcinogen which implies a carcinogenic risk to humans. In patients receiving long-term hydroxyurea for myeloproliferative disorders, such as polycythemia vera and thrombocythemia, secondary leukemias have been reported. It is unknown whether this leukemogenic effect is secondary to hydroxyurea or is associated with the patient’s underlying disease. The physician and patient must very carefully consider the potential benefits of Hydroxyurea relative to the undefined risk of developing secondary malignancies.
DESCRIPTION
Hydroxyurea capsules is available for oral use as capsules providing 200 mg, 300 mg, and 400 mg hydroxyurea. Inactive ingredients: citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colorants; FD&C Blue No. 1 and FD&C Green No. 3 (200 mg capsules); D&C Red No. 28, D&C Red No. 33, and FD&C Blue No. 1 (300 mg capsules); D&C Red No. 28, D&C Red No. 33, and D&C Yellow No. 10 (400 mg capsules).
Hydroxyurea is an essentially tasteless, white crystalline powder. Its structural formula is:
CLINICAL PHARMACOLOGY
Mechanism of ActionThe precise mechanism by which hydroxyurea produces its cytotoxic and cytoreductive effects is not known. However, various studies support the hypothesis that hydroxyurea causes an immediate inhibition of DNA synthesis by acting as a ribonucleotide reductase inhibitor, without interfering with the synthesis of ribonucleic acid or of protein.
The mechanisms by which Hydroxyurea produces its beneficial effects in patients with sickle cell anemia (SCA) are uncertain. Known pharmacologic effects of Hydroxyurea that may contribute to its beneficial effects include increasing hemoglobin F levels in RBCs, decreasing neutrophils, increasing the water content of RBCs, increasing deformability of sickled cells, and altering the adhesion of RBCs to endothelium.
Hydroxyurea is readily absorbed after oral administration. Peak plasma levels are reached in 1 to 4 hours after an oral dose. With increasing doses, disproportionately greater mean peak plasma concentrations and AUCs are observed.
There are no data on the effect of food on the absorption of hydroxyurea.
Hydroxyurea distributes rapidly and widely in the body with an estimated volume of distribution approximating total body water.
Plasma to ascites fluid ratios range from 2:1 to 7.5:1. Hydroxyurea concentrates in leukocytes and erythrocytes.
Up to 60% of an oral dose undergoes conversion through metabolic pathways that are not fully characterized. One pathway is probably saturable hepatic metabolism. Another minor pathway may be degradation by urease found in intestinal bacteria. Acetohydroxamic acid was found in the serum of three leukemic patients receiving hydroxyurea and may be formed from hydroxylamine resulting from action of urease on hydroxyurea.
ExcretionExcretion of hydroxyurea in humans is likely a linear first-order renal process. In adults with SCA, mean cumulative urinary recovery of hydroxyurea was about 40% of the administered dose.
Special PopulationsGeriatric, Gender, Race
No information is available regarding pharmacokinetic differences due to age, gender, or race.
PediatricNo pharmacokinetic data are available in pediatric patients treated with hydroxyurea for SCA.
Renal InsufficiencyAs renal excretion is a pathway of elimination, consideration should be given to decreasing the dosage of hydroxyurea in patients with renal impairment. In adult patients with sickle cell disease, an open-label, non-randomized, single-dose, multicenter study was conducted to assess the influence of renal function on the pharmacokinetics of hydroxyurea. Patients in the study with normal renal function (creatinine clearance [CrCl] >80 mL/min), mild (CrCl 50-80 mL/min), moderate (CrCl = 30-<50 mL/min), or severe (<30 mL/min) renal impairment received hydroxyurea as a single oral dose of 15 mg/kg, achieved by using combinations of the 200 mg, 300 mg, or 400 mg capsules. Patients with end-stage renal disease (ESRD) received two doses of 15 mg/kg separated by 7 days, the first was given following a 4-hour hemodialysis session, the second prior to hemodialysis. In this study, the mean exposure (AUC) in patients whose creatinine clearance was <60 mL/min (or ESRD) was approximately 64% higher than in patients with normal renal function. The results suggest that the initial dose of hydroxyurea should be reduced when used to treat patients with renal impairment. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.) The table below describes the recommended dosage modification.
Creatinine Clearance (mL/min) |
Recommended Hydroxyurea Initial Dose |
≥60 |
15 |
*On dialysis days, hydroxyurea should be administered to patients with ESRD following hemodialysis.
Close monitoring of hematologic parameters is advised in these patients.
Hepatic InsufficiencyThere are no data that support specific guidance for dosage adjustment in patients with hepatic impairment. Close monitoring of hematologic parameters is advised in these patients.
Drug InteractionsThere are no data on concomitant use of hydroxyurea with other drugs in humans.
Clinical Studies
The efficacy of hydroxyurea in sickle cell anemia was assessed in a large clinical study (Multicenter Study of Hydroxyurea in Sickle Cell Anemia).1
The study was a randomized, double-blind, placebo-controlled trial that evaluated 299 adult patients (≥18 years) with moderate to severe disease (≥3 painful crises yearly). The trial was stopped by the Data Safety Monitoring Committee, after accrual was completed but before the scheduled 24 months of follow-up was completed in all patients, based on observations of fewer painful crises among patients receiving hydroxyurea.
Compared to placebo treatment, treatment with hydroxyurea resulted in a significant decrease in the yearly rate of painful crises, the yearly rate of painful crises requiring hospitalization, the incidence of chest syndrome, the number of patients transfused, and units of blood transfused. Hydroxyurea treatment significantly increased the median time to both first and second painful crises.
Although patients with 3 or more painful crises during the preceding 12 months were eligible for the study, most of the benefit in crisis reduction was seen in the patients with 6 or more painful crises during the preceding 12 months.
EVENT |
HYDROXYUREA |
PLACEBO |
PERCENT CHANGE |
P-VALUE |
Median yearly rate of painful crises* |
2.5 |
4.6 |
−46 |
= 0.001 |
Median yearly rate of painful crises requiring hospitalization |
1 |
2.5 |
−60 |
= 0.0027 |
Median time to first painful crisis (months) |
2.76 |
1.35 |
104 |
= 0.014 |
Median time to second painful crisis (months) |
6.58 |
4.13 |
59 |
= 0.0024 |
Incidence of chest syndrome (# episodes) |
56 |
101 |
−45 |
= 0.003 |
Number of patients transfused |
55 |
79 |
−30 |
= 0.002 |
Number of units of blood transfused |
423 |
670 |
−37 |
= 0.003 |
* A painful crisis was defined in the study as acute sickling-related pain that resulted in a visit to a medical facility, that lasted more than 4 hours, and that required treatment with a parenteral narcotic or NSAID. Chest syndrome, priapism, and hepatic sequestration were also included in this definition.
No deaths were attributed to treatment with hydroxyurea, and none of the patients developed neoplastic disorders during the study. Treatment was permanently stopped for medical reasons in 14 hydroxyurea-treated (2 patients with myelotoxicity) and 6 placebo-treated patients.
Fetal HemoglobinIn patients with SCA treated with hydroxyurea, fetal hemoglobin (HbF) increases 4 to 12 weeks after initiation of treatment. In general, average HbF levels correlate with dose and plasma level with possible plateauing at higher dosages.
A clear relation between reduction in crisis frequency and increased HbF or F-cell levels has not been demonstrated. The dose-related cytoreductive effects of hydroxyurea, particularly on neutrophils, was the factor most strongly correlated with reduced crisis frequency.
INDICATIONS AND USAGE
Hydroxyurea is indicated to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult patients with sickle cell anemia with recurrent moderate to severe painful crises (generally at least 3 during the preceding 12 months).
CONTRAINDICATIONS
Hydroxyurea is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of its formulation.
WARNINGSHydroxyurea is a cytotoxic and myelosuppressive agent. Hydroxyurea should not be given if bone marrow function is markedly depressed, as indicated by neutrophils below 2000 cells/mm3; a platelet count below 80,000/mm3; a hemoglobin level below 4.5 g/dL; or reticulocytes below 80,000/mm3 when the hemoglobin concentration is below 9 g/dL. Neutropenia is generally the first and most common manifestation of hematologic suppression. Thrombocytopenia and anemia occur less often, and are seldom seen without a preceding leukopenia. Recovery from myelosuppression is usually rapid when therapy is interrupted. Hydroxyurea causes macrocytosis, which may mask the incidental development of folic acid deficiency. Prophylactic administration of folic acid is recommended.
In HIV-infected patients during therapy with hydroxyurea and didanosine, with or without stavudine, fatal and nonfatal pancreatitis have occurred. Hepatotoxicity and hepatic failure resulting in death have been reported during postmarketing surveillance in HIV-infected patients treated with hydroxyurea and other antiretroviral agents. Fatal hepatic events were reported most often in patients treated with the combination of hydroxyurea, didanosine, and stavudine. This combination should be avoided.
Peripheral neuropathy, which was severe in some cases, has been reported in HIV-infected patients receiving hydroxyurea in combination with antiretroviral agents, including didanosine, with or without stavudine.
Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients with myeloproliferative disorders during therapy with hydroxyurea. These vasculitic toxicities were reported most often in patients with a history of, or currently receiving, interferon therapy. Due to potentially severe clinical outcomes for the cutaneous vasculitic ulcers reported in patients with myeloproliferative disease, hydroxyurea should be discontinued if cutaneous vasculitic ulcerations develop.
Carcinogenesis and Mutagenesis(See BOXED WARNING.)
Hydroxyurea is genotoxic in a wide range of test systems and is thus presumed to be a human carcinogen. In patients receiving long-term hydroxyurea for myeloproliferative disorders, such as polycythemia vera and thrombocythemia, secondary leukemia has been reported. It is unknown whether this leukemogenic effect is secondary to hydroxyurea or is associated with the patient’s underlying disease. Skin cancer has also been reported in patients receiving long-term hydroxyurea.
Conventional long-term studies to evaluate the carcinogenic potential of Hydroxyurea have not been performed. However, intraperitoneal administration of 125 to 250 mg/kg hydroxyurea (about 0.6-1.2 times the maximum recommended human oral daily dose on a mg/m2 basis) thrice weekly for 6 months to female rats increased the incidence of mammary tumors in rats surviving to 18 months compared to control. Hydroxyurea is mutagenic in vitro to bacteria, fungi, protozoa, and mammalian cells. Hydroxyurea is clastogenic in vitro (hamster cells, human lymphoblasts) and in vivo (SCE assay in rodents, mouse micronucleus assay). Hydroxyurea causes the transformation of rodent embryo cells to a tumorigenic phenotype.
PregnancyHydroxyurea can cause fetal harm when administered to a pregnant woman. Hydroxyurea has been demonstrated to be a potent teratogen in a wide variety of animal models, including mice, hamsters, cats, miniature swine, dogs, and monkeys at doses within 1-fold of the human dose given on a mg/m2 basis. Hydroxyurea is embryotoxic and causes fetal malformations (partially ossified cranial bones, absence of eye sockets, hydrocephaly, bipartite sternebrae, missing lumbar vertebrae) at 180 mg/kg/day (about 0.8 times the maximum recommended human daily dose on a mg/m2 basis) in rats and at 30 mg/kg/day (about 0.3 times the maximum recommended human daily dose on a mg/m2 basis) in rabbits. Embryotoxicity was characterized by decreased fetal viability, reduced live litter sizes, and developmental delays. Hydroxyurea crosses the placenta. Single doses of ≥375 mg/kg (about 1.7 times the maximum recommended human daily dose on a mg/m2 basis) to rats caused growth retardation and impaired learning ability. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
PRECAUTIONSGeneral
Therapy with Hydroxyurea requires close supervision. Some patients treated at the recommended initial dose of 15 mg/kg/day have experienced severe or life-threatening myelosuppression, requiring interruption of treatment and dose reduction. The hematologic status of the patient, as well as kidney and liver function should be determined prior to, and repeatedly during treatment. Treatment should be interrupted if neutrophil levels fall to <2000/mm3; platelets fall to <80,000/mm3; hemoglobin declines to less than 4.5 g/dL; or if reticulocytes fall below 80,000/mm3 when the hemoglobin concentration is below 9 g/dL. Following recovery, treatment may be resumed at lower doses (see DOSAGE AND ADMINISTRATION).
Hydroxyurea should be used with caution in patients with renal dysfunction. Data from a single-dose study of the pharmacokinetics of hydroxyurea in patients with sickle cell anemia suggest that the initial dose of hydroxyurea should be reduced in patients with renal impairment. (See CLINICAL PHARMACOLOGY: Special Populations and DOSAGE AND ADMINISTRATION.)
Patients must be able to follow directions regarding drug administration and their monitoring and care.
Hydroxyurea is not indicated for the treatment of HIV infection; however, if HIV-infected patients are treated with hydroxyurea, and in particular, in combination with didanosine and/or stavudine, close monitoring for signs and symptoms of pancreatitis is recommended. Patients who develop signs and symptoms of pancreatitis should permanently discontinue therapy with hydroxyurea.
An increased risk of hepatotoxicity, which may be fatal, may occur in patients treated with hydroxyurea, and in particular, in combination with didanosine and stavudine. This combination should be avoided.
See WARNINGS and BOXED WARNING for Carcinogenesis and Mutagenesis information.
Impairment of Fertility: Hydroxyurea administered to male rats at 60 mg/kg/day (about 0.3 times the maximum recommended human daily dose on a mg/m2 basis) produced testicular atrophy, decreased spermatogenesis, and significantly reduced their ability to impregnate females.
PregnancyPregnancy Category D. (See WARNINGS.)
Nursing Mothers
Hydroxyurea is excreted in human milk. Because of the potential for serious adverse reactions with hydroxyurea, a decision should be made either to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric UseSafety and effectiveness in pediatric patients have not been established.
Drug InteractionsProspective studies on the potential for hydroxyurea to interact with other drugs have not been performed.
Studies have shown that there is an analytical interference of hydroxyurea with the enzymes (urease, uricase, and lactate dehydrogenase) used in the determination of urea, uric acid and lactic acid, rendering falsely elevated results of these in patients treated with hydroxyurea.
Information for Patients
(See Patient Information at end of labeling.)
Patients should be reminded that this medication must be handled with care. People who are not taking Hydroxyurea should not be exposed to it. To decrease the risk of exposure, wear disposable gloves when handling Hydroxyurea or bottles containing Hydroxyurea . Anyone handling Hydroxyurea should wash their hands before and after contact with the bottle or capsules. If the powder from the capsule is spilled, it should be wiped up immediately with a damp disposable towel and discarded in a closed container, such as a plastic bag. The medication should be kept away from children and pets. Contact your doctor for instructions on how to dispose of outdated capsules.
The necessity of monitoring blood counts every two weeks, throughout the duration of therapy, should be emphasized.
ADVERSE REACTIONS
Sickle Cell AnemiaIn patients treated for sickle cell anemia in the Multicenter Study of Hydroxyurea in Sickle Cell Anemia,1 the most common adverse reactions were hematologic, with neutropenia, and low reticulocyte and platelet levels necessitating temporary cessation in almost all patients. Hematologic recovery usually occurred in two weeks.
Non-hematologic events that possibly were associated with treatment include hair loss, skin rash, fever, gastrointestinal disturbances, weight gain, bleeding, and parvovirus B-19 infection; however, these non-hematologic events occurred with similar frequencies in the hydroxyurea and placebo treatment groups. Melanonychia has also been reported in patients receiving Hydroxyurea for SCA.
OtherAdverse events associated with the use of hydroxyurea in the treatment of neoplastic diseases, in addition to hematologic effects include: gastrointestinal symptoms (stomatitis, anorexia, nausea, vomiting, diarrhea, and constipation), and dermatological reactions such as maculopapular rash, skin ulceration, dermatomyositis-like skin changes, peripheral erythema, and facial erythema. Hyperpigmentation, atrophy of skin and nails, scaling, and violet papules have been observed in some patients after several years of long-term daily maintenance therapy with hydroxyurea. Skin cancer has been reported. Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients with myeloproliferative disorders during therapy with hydroxyurea. These vasculitic toxicities were reported most often in patients with a history of, or currently receiving, interferon therapy (see WARNINGS). Dysuria and alopecia have been reported. Large doses may produce drowsiness. Neurological disturbances have occurred and were limited to headache, dizziness, disorientation, hallucinations, and convulsions. Hydroxyurea may cause temporary impairment of renal tubular function accompanied by elevations in serum uric acid, blood urea nitrogen (BUN), and creatinine levels. Abnormal bromsulphalein (BSP) retention has been reported. Fever, chills, malaise, edema, asthenia, and elevation of hepatic enzymes have also been reported.
The association of hydroxyurea with the development of acute pulmonary reactions consisting of diffuse pulmonary infiltrates, fever, and dyspnea has been reported. Pulmonary fibrosis also has been reported.
In HIV-infected patients who received hydroxyurea in combination with antiretroviral agents, in particular, didanosine plus stavudine, fatal and nonfatal pancreatitis and hepatotoxicity, and severe peripheral neuropathy have been reported. Patients treated with hydroxyurea in combination with didanosine, stavudine, and indinavir in Study ACTG 5025 showed a median decline in CD4 cells of approximately 100/mm3.
OVERDOSAGE
Acute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at dosages several times the therapeutic dose. Soreness, violet erythema, edema on palms and soles followed by scaling of hands and feet, severe generalized hyperpigmentation of the skin, and stomatitis have been observed.
DOSAGE AND ADMINISTRATION
Procedures for proper handling and disposal of cytotoxic drugs should be considered. Several guidelines on this subject have been published.2-5
To minimize the risk of dermal exposure, always wear impervious gloves when handling bottles containing Hydroxyurea capsules. Hydroxyurea capsules should not be opened. Personnel should avoid exposure to crushed or opened capsules. If contact with crushed or opened capsules occurs, wash immediately and thoroughly. More information is available in the references listed below.
Dosage should be based on the patient’s actual or ideal weight, whichever is less. The initial dose of Hydroxyurea is 15 mg/kg/day as a single dose. The patient’s blood count must be monitored every two weeks. (See WARNINGS.)
If blood counts are in an acceptable range*, the dose may be increased by 5 mg/kg/day every 12 weeks until a maximum tolerated dose (the highest dose that does not produce toxic** blood counts over 24 consecutive weeks), or 35 mg/kg/day, is reached.
If blood counts are between the acceptable range* and toxic**, the dose is not increased.
If blood counts are considered toxic**, Hydroxyurea should be discontinued until hematologic recovery. Treatment may then be resumed after reducing the dose by 2.5 mg/kg/day from the dose associated with hematologic toxicity. Hydroxyurea may then be titrated up or down, every 12 weeks in 2.5 mg/kg/day increments, until the patient is at a stable dose that does not result in hematologic toxicity for 24 weeks. Any dosage on which a patient develops hematologic toxicity twice should not be tried again.
*acceptable range =
neutrophils ≥2500 cells/mm3,
platelets ≥95,000/mm3,
hemoglobin >5.3 g/dL and
reticulocytes ≥95,000/mm3 if the hemoglobin concentration <9 g/dL.
**toxic =
neutrophils <2000 cells/mm3,
platelets <80,000/mm3,
hemoglobin <4.5 g/dL and
reticulocytes <80,000/mm3 if the hemoglobin concentration <9 g/dL.
Since hydroxyurea may raise the serum uric acid level, dosage adjustment of uricosuric medication may be necessary.
As renal excretion is a pathway of elimination, consideration should be given to decreasing the dosage of Hydroxyurea in patients with renal impairment. The results of a single-dose study of the influence of renal function on the pharmacokinetics of hydroxyurea in adults with sickle cell disease suggest that the initial dose of hydroxyurea should be reduced by 50%, to 7.5 mg/kg/day, when used to treat patients with renal impairment. (See PRECAUTIONS and CLINICAL PHARMACOLOGY.) Close monitoring of hematologic parameters is advised in these patients.
Recommended Hydroxyurea Initial Dose |
|
≥60 |
15 |
*On dialysis days, hydroxyurea should be administered to patients with ESRD following hemodialysis.
Hepatic InsufficiencyThere are no data that support specific guidance for dosage adjustment in patients with hepatic impairment. Close monitoring of hematologic parameters is advised in these patients.
HOW SUPPLIED
THAROLAX ® (hydroxyurea capsules, USP).
200 mg capsules packaged in HDPE bottles of 60 with a plastic safety screw cap. The cap and body are opaque blue-green. The capsule is marked in black ink on both the cap and body with “TAJ” and “200”.
300 mg capsules packaged in HDPE bottles of 60 with a plastic safety screw cap. The cap and body are opaque purple. The capsule is marked in black ink on both the cap and body with “TAJ” and “300”.
400 mg capsules packaged in HDPE bottles of 60 with a plastic safety screw cap. The cap and body are opaque reddish-orange. The capsule is marked in black ink on both the cap and body with “TAJ” and “400”.
Storage
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Keep tightly closed.
REFERENCES- Charache S, Barton FB, Moore RD, et al; Hydroxyurea and sickle cell anemia: clinical utility of a myelosuppressive “switching” agent. Medicine. 1996;75:300-326.
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich M, White JM, Kelleher LO, eds. 2005. Chemotherapy and biotherapy guidelines and recommendations for practice. 2nd ed. Pittsburgh, PA: Oncology Nursing Society.
Manufactured by:
THAROLAX ® capsules is made in India by:Taj Pharma India Brands
Taj Pharma Group (India)
214, Bake House, Bake House Lane,
Fort, Mumbai 400001, India.
Phone:+91-22-2637-4592 / 93
General EPA Bx:+91-84484-44095
Toll Free Number: 1800-222-434 / 1800-222-825
Fax:+91-22-2634-1274
E-mail:tharolax@tajpharma.com
Product Glimpse
Description
Hydroxyurea is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults with sickle cell anemia. How Hydroxyurea works is not certain but it may work by reducing the number of white blood cells and/or increasing red blood cells that carry fetal hemoglobin (HbF). Fetal hemoglobin may prevent sickling.
Hydroxyurea is used to treat various types of cancers such as melanoma, leukemia and cancer of the ovary. Hydroxyurea contains hydroxyurea, an anti-cancer medicine. It interferes with the replication of cells and causes cell death, particularly in cancer cells.