Lenalidomide 5mg/10mg Capsules
Lenalidomide
Lenalidomide 5mg/10mg Capsules
White and blue-green opaque hard capsules imprinted “TAJ” on one half and “2.5 mg” on the other half in black ink:
2.5 mg bottles of 28
2.5 mg bottles of 100
White opaque capsules imprinted “TAJ” on one half and “5 mg” on the other half in black ink:
5 mg bottles of 28
5 mg bottles of 100
Blue/green and pale yellow opaque capsules imprinted “TAJ” on one half and “10 mg” on the other half in black ink:
10 mg bottles of 28
10 mg bottles of 100
Powder blue and white opaque capsules imprinted “TAJ” on one half and “15 mg” on the other half in black ink:
15 mg bottles of 21
15 mg bottles of 100
Powder blue and blue-green opaque hard capsules imprinted “TAJ” on one half and “20 mg” on the other half in black ink.
20 mg bottles of 21
20 mg bottles of 100
White opaque capsules imprinted “TAJ” on one half and “25 mg” on the other half in black ink:
25 mg bottles of 21
25 mg bottles of 100
Storage
Store at 20°C - 25°C (68°F - 77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [See USP Controlled Room Temperature].
Handling and Disposal
Care should be exercised in the handling of Lenalidomide. Lenalidomide capsules should not be opened or crushed. If powder from Lenalidomide contacts the skin, wash the skin immediately and thoroughly with soap and water. If Lenalidomide contacts the mucous membranes, flush thoroughly with water.
Procedures for the proper handling and disposal of anticancer drugs should be considered. Several guidelines on the subject have been published.1
Dispense no more than a 28-day supply.
Lenalidomide
Lenalidomide 5mg/10mg Capsules
What is Lenalidomide?
Lenalidomide is a prescription medicine used to treat people:
- with multiple myeloma (MM) who have received at least one prior medicine, taken along with the medicine dexamethasone.
- who have a condition called myelodysplastic syndromes (MDS). Lenalidomide is for the type of MDS with a chromosome problem where part of chromosome 5 is missing. This type of MDS is known as deletion 5q MDS. People with this type of MDS may have low red blood cell counts that require treatment with blood transfusions.
- with mantle cell lymphoma (MCL) when the disease comes back or becomes worse after treatment with two prior medicines, one of which included bortezomib. Mantle cell lymphoma is a cancer of a type of white blood cell called lymphocytes that are in the lymph nodes.
Lenalidomide should not be used to treat people who have chronic lymphocytic leukemia (CLL) unless they are participants in a controlled clinical trial.
It is not known if Lenalidomide is safe and effective in children under 18 years of age.
Who should not take Lenalidomide?
- Do not take Lenalidomide if you are pregnant, plan to become pregnant, or become pregnant during Lenalidomide treatment.
- Do not take Lenalidomide if you are allergic to lenalidomide or any of the ingredients in Lenalidomide. See the end of this Medication Guide for a complete list of ingredients in Lenalidomide.
What should I tell my healthcare provider before taking Lenalidomide?
Before you take Lenalidomide, tell your healthcare provider if you:
- have liver problems
- are lactose intolerant. Lenalidomide contains lactose.
- have any other medical condition
- are breastfeeding. Lenalidomide must not be used by females who are breastfeeding. It is not known if Lenalidomide passes into your breast milk and can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Lenalidomide and other medicines may affect each other causing serious side effects.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist.
How should I take Lenalidomide?
Take Lenalidomide exactly as prescribed and follow all the instructions of the Lenalidomide REMS™ program.
Before prescribing Lenalidomide, your healthcare provider will:
- explain the Lenalidomide REMS™ program to you
- have you sign the Patient-Physician Agreement Form
- Swallow Lenalidomide capsules whole with water 1 time a day. Do not break, chew, or open your capsules.
- Take Lenalidomide at about the same time each day.
- Do not open the Lenalidomide capsules or handle them any more than needed. If you touch a broken Lenalidomide capsule or the medicine in the capsule, wash the area of your body with soap and water.
- If you miss a dose of Lenalidomide, and it has been less than 12 hours since your regular time, take it as soon as you remember. If it has been more than 12 hours, just skip your missed dose. Do not take 2 doses at the same time.
- If you take too much Lenalidomide or overdose, call your healthcare provider right away.
Females who can become pregnant:
- will have pregnancy tests weekly for 4 weeks, then every 4 weeks if your menstrual cycle is regular, or every 2 weeks if your menstrual cycle is irregular.
- If you miss your period or have unusual bleeding, you will need to have a pregnancy test and receive counseling.
- must agree to use 2 different forms of effective birth control at the same time every time, for 4 weeks before, while taking, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping Lenalidomide.
Males who take Lenalidomide, even those who have had a vasectomy, must agree to use a latex or synthetic condom during sexual contact with a pregnant female or a female who can become pregnant.
What should I avoid while taking Lenalidomide?
- Females: Do not get pregnant and do not breastfeed while taking Lenalidomide.
- Males: Do not donate sperm
- Do not share Lenalidomide with other people. It may cause birth defects and other serious problems.
- Do not donate blood while you take Lenalidomide, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping Lenalidomide. If someone who is pregnant gets your donated blood, her baby may be exposed to Lenalidomide and may be born with birth defects.
What are the possible side effects of Lenalidomide?
Lenalidomide may cause serious side effects, including:
- Increased risk of death in people who have chronic lymphocytic leukemia (CLL). People with CLL who take Lenalidomide have an increased risk of death compared with people who take the medicine chlorambucil. Lenalidomide may cause you to have serious heart problems that can lead to death, including atrial fibrillation, heart attack, or heart failure. You should not take Lenalidomide if you have CLL unless you are participating in a controlled clinical trial.
- Risk of new cancers (malignancies). People with multiple myeloma who receive melphalan (a type of chemotherapy) and a blood stem cell transplant with the addition of Lenalidomide have a higher risk of developing new cancers, including certain blood cancers (acute myelogenous leukemia or AML) and a type of lymphoma called Hodgkin lymphoma. Talk with your healthcare provider about your risk of developing new cancers if you take Lenalidomide. Your healthcare provider will check you for new cancers during your treatment with Lenalidomide.
- Severe liver problems, including liver failure and death. Tell your healthcare provider right away if you develop any of the following symptoms of liver problems:
- yellowing of your skin or the white part of your eyes (jaundice)
- dark or brown (tea colored) urine
- pain on the upper right side of your stomach area (abdomen)
- bleeding or bruising more easily than normal
- feeling very tired
Your healthcare provider will do blood tests to check your liver function during your treatment with Lenalidomide.
- Serious skin reactions. Serious skin reactions can happen with Lenalidomide and may cause death. Call your healthcare provider right away if you have any skin reaction while taking Lenalidomide.
- Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
- Worsening of your tumor (tumor flare reaction). Tell your healthcare provider if you get any of these symptoms of tumor flare reaction while taking Lenalidomide: tender swollen lymph nodes, low-grade fever, pain, or rash.
Lenalidomide may cause serious side effects, including:
Common side effects of Lenalidomide include:
- diarrhea
- itching
- rash
- tiredness
These are not all the possible side effects of Lenalidomide.
Call your doctor for medical advice about side effects. You may report side effects to FDA
How should I store Lenalidomide?
- Store Lenalidomide at room temperature between 68°F to 77°F (20°C to 25°C).
- Return any unused Lenalidomide to Celgene or your healthcare provider.
Keep Lenalidomide and all medicines out of the reach of children.
General information about Lenalidomide
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take Lenalidomide for conditions for which it was not prescribed. Do not give Lenalidomide to other people, even if they have the same symptoms you have. It may harm them and may cause birth defects.
If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Lenalidomide that is written for health professionals.
What are the ingredients in Lenalidomide?
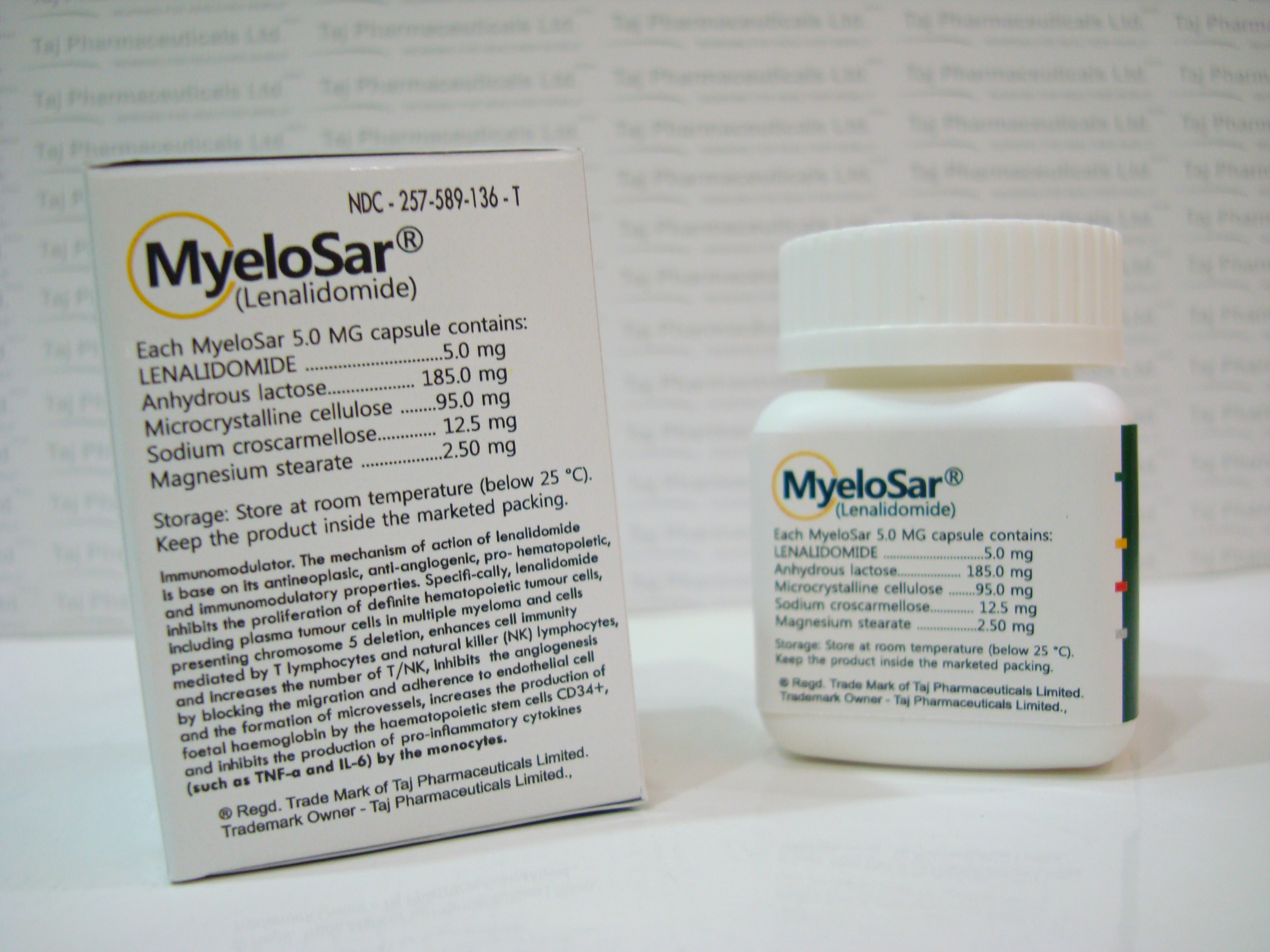
Active ingredient: lenalidomide
Inactive ingredients: lactose anhydrous, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate.
The 5 mg and 25 mg capsule shells contain gelatin, titanium dioxide and black ink. The 2.5 and 10 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink. The 15 mg capsule shell contains gelatin, FD&C blue #2, titanium dioxide and black ink. The 20 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink.
Lenalidomide
Lenalidomide 5mg/10mg Capsules
INDICATIONS AND USAGE
Multiple Myeloma
Lenalidomide in combination with dexamethasone is indicated for the treatment of patients with multiple myeloma (MM) who have received at least one prior therapy.
Myelodysplastic Syndromes
Lenalidomide is indicated for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities.
Mantle Cell Lymphoma
Lenalidomide is indicated for the treatment of patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib.
Limitations of Use
Lenalidomide is not indicated and is not recommended for the treatment of patients with CLL outside of controlled clinical trials.
DOSAGE AND ADMINISTRATION
Lenalidomide should be taken orally at about the same time each day, either with or without food. Lenalidomide capsules should be swallowed whole with water. The capsules should not be opened, broken, or chewed.
Multiple Myeloma
The recommended starting dose of Lenalidomide is 25 mg once daily on Days 1-21 of repeated 28-day cycles. The recommended dose of dexamethasone is 40 mg once daily on Days 1-4, 9-12, and 17-20 of each 28-day cycle for the first 4 cycles of therapy and then 40 mg once daily orally on Days 1-4 every 28 days. Treatment is continued or modified based upon clinical and laboratory findings.
Dose Adjustments for Hematologic Toxicities During Multiple Myeloma Treatment
Dose modification guidelines, as summarized below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to Lenalidomide.
| When Platelets | Recommended Course |
|---|---|
| Fall to <30,000/mcL | Interrupt Lenalidomide treatment, follow CBC weekly |
| Return to =30,000/mcL | Restart Lenalidomide at 15 mg daily |
| For each subsequent drop <30,000/mcL | Interrupt Lenalidomide treatment |
| Return to =30,000/mcL | Resume Lenalidomide at 5 mg less than the previous dose. Do not dose below 5 mg daily |
| When Neutrophils | Recommended Course |
|---|---|
| Fall to <1000/mcL | Interrupt Lenalidomide treatment, add G-CSF, follow CBC weekly |
| Return to =1,000/mcL and neutropenia is the only toxicity | Resume Lenalidomide at 25 mg daily |
| Return to =1,000/mcL and if other toxicity | Resume Lenalidomide at 15 mg daily |
| For each subsequent drop <1,000/mcL | Interrupt Lenalidomide treatment |
| Return to =1,000/mcL | Resume Lenalidomide at 5 mg less than the previous dose. Do not dose below 5 mg daily |
Other Grade 3 / 4 Toxicities in MM
For other Grade 3/4 toxicities judged to be related to Lenalidomide, hold treatment and restart at the physician's discretion at next lower dose level when toxicity has resolved to = Grade 2.
Starting Dose Adjustment for Renal Impairment in MM:
See Section 2.4
Myelodysplastic Syndromes
The recommended starting dose of Lenalidomide is 10 mg daily. Treatment is continued or modified based upon clinical and laboratory findings.
Dose Adjustments for Hematologic Toxicities During MDS Treatment
Patients who are dosed initially at 10 mg and who experience thrombocytopenia should have their dosage adjusted as follows:
| If baseline =100,000/mcL | |
|---|---|
| When Platelets | Recommended Course |
| Fall to <50,000/mcL | Interrupt Lenalidomide treatment |
| Return to =50,000/mcL | Resume Lenalidomide at 5 mg daily |
| If baseline <100,000/mcL | |
| When Platelets | Recommended Course |
| Fall to 50% of the baseline value | Interrupt Lenalidomide treatment |
| If baseline =60,000/mcL and returns to =50,000/mcL | Resume Lenalidomide at 5 mg daily |
| If baseline <60,000/mcL and returns to =30,000/mcL | Resume Lenalidomide at 5 mg daily |
If thrombocytopenia develops AFTER 4 weeks of starting treatment at 10 mg daily in MDS
| When Platelets | Recommended Course |
|---|---|
| <30,000/mcL or <50,000/mcL with platelet transfusions | Interrupt Lenalidomide treatment |
| Return to =30,000/mcL (without hemostatic failure) | Resume Lenalidomide at 5 mg daily |
Patients who experience thrombocytopenia at 5 mg daily should have their dosage adjusted as follows:
If thrombocytopenia develops during treatment at 5 mg daily in MDS
| When Platelets | Recommended Course |
|---|---|
| <30,000/mcL or <50,000/mcL with platelet transfusions | Interrupt Lenalidomide treatment |
| Return to =30,000/mcL (without hemostatic failure) | Resume Lenalidomide at 2.5 mg daily |
Patients who are dosed initially at 10 mg and experience neutropenia should have their dosage adjusted as follows:
Absolute Neutrophil counts (ANC)
If neutropenia develops WITHIN 4 weeks of starting treatment at 10 mg daily in MDS
| If baseline ANC =1,000/mcL | |
|---|---|
| When Neutrophils | Recommended Course |
| Fall to <750/mcL | Interrupt Lenalidomide treatment |
| Return to =1,000/mcL | Resume Lenalidomide at 5 mg daily |
| If baseline ANC <1,000/mcL | |
| When Neutrophils | Recommended Course |
| Fall to <500/mcL | Interrupt Lenalidomide treatment |
| Return to =500/mcL | Resume Lenalidomide at 5 mg daily |
If neutropenia develops AFTER 4 weeks of starting treatment at 10 mg daily in MDS
| When Neutrophils | Recommended Course |
|---|---|
| <500/mcL for =7 days or <500/mcL associated with fever (=38.5°C) | Interrupt Lenalidomide treatment |
| Return to =500/mcL | Resume Lenalidomide at 5 mg daily |
Patients who experience neutropenia at 5 mg daily should have their dosage adjusted as follows:
If neutropenia develops during treatment at 5 mg daily in MDS
| When Neutrophils | Recommended Course |
|---|---|
| <500/mcL for =7 days or <500/mcL associated with fever (=38.5°C) | Interrupt Lenalidomide treatment |
| Return to =500/mcL | Resume Lenalidomide at 2.5 mg daily |
Other Grade 3 / 4 Toxicities in MDS
For other Grade 3/4 toxicities judged to be related to Lenalidomide, hold treatment and restart at the physician's discretion at next lower dose level when toxicity has resolved to = Grade 2.
Starting Dose Adjustment for Renal Impairment in MDS:
See Section 2.4
Mantle Cell Lymphoma
The recommended starting dose of Lenalidomide is 25 mg/day orally on Days 1-21 of repeated 28-day cycles for relapsed or refractory mantle cell lymphoma. Treatment should be continued until disease progression or unacceptable toxicity.
Treatment is continued, modified or discontinued based upon clinical and laboratory findings.
Dose Adjustments for Hematologic Toxicities During MCL Treatment
Dose modification guidelines as summarized below are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicities considered to be related to Lenalidomide.
| When Platelets | Recommended Course |
|---|---|
| Fall to <50,000/mcL | Interrupt Lenalidomide treatment and follow CBC weekly |
| Return to =50,000/mcL | Resume Lenalidomide at 5 mg less than the previous dose. Do not dose below 5 mg daily |
| When Neutrophils | Recommended Course |
|---|---|
| Fall to <1000/mcL for at least 7 days OR Falls to < 1,000/mcL with an associated temperature = 38.5°C OR Falls to < 500 /mcL | Interrupt Lenalidomide treatment and follow CBC weekly |
| Return to =1,000/mcL | Resume Lenalidomide at 5 mg less than the previous dose. Do not dose below 5 mg daily |
Other Grade 3 / 4 Toxicities in MCL
For other Grade 3/4 toxicities judged to be related to Lenalidomide, hold treatment and restart at the physician’s discretion at next lower dose level when toxicity has resolved to = Grade 2.
Starting Dose Adjustment for Renal Impairment in MCL:
See Section 2.4
Starting Dose for Renal Impairment in MM, MDS or MCL
Since Lenalidomide is primarily excreted unchanged by the kidney, adjustments to the starting dose of Lenalidomide are recommended to provide appropriate drug exposure in patients with moderate or severe renal impairment and in patients on dialysis. Based on a pharmacokinetic study in patients with renal impairment due to non-malignant conditions, Lenalidomide starting dose adjustment is recommended for patients with CLcr < 60 mL/min. Non-dialysis patients with creatinine clearances less than 11 mL/min and dialysis patients with creatinine clearances less than 7 mL/min have not been studied. The recommendations for initial starting doses for patients with MM, MDS or MCL are as follows:
| Category | Renal Function (Cockcroft- Gault) | Dose in MM or MCL | Dose in MDS |
|---|---|---|---|
| Moderate Renal Impairment | CLcr 30-60 mL/min | 10 mg Every 24 hours | 5 mg Every 24 hours |
| Severe Renal Impairment | CLcr < 30 mL/min (not requiring dialysis) | 15 mg Every 48 hours | 2.5 mg Every 24 hours |
| End Stage Renal Disease | CLcr < 30 mL/min (requiring dialysis) | 5 mg Once daily. On dialysis days, administer the dose following dialysis. | 2.5 mg Once daily. On dialysis days, administer the dose following dialysis. |
After initiation of Lenalidomide therapy, subsequent Lenalidomide dose modification is based on individual patient treatment tolerance, as described elsewhere.
DOSAGE FORMS AND STRENGTHS
Lenalidomide 2.5 mg, 5 mg, 10 mg, 15 mg, 20 mg and 25 mg capsules will be supplied through the Lenalidomide REMS™ program.
Lenalidomide is available in the following capsule strengths:
2.5 mg: White and blue-green opaque hard capsules imprinted “REV” on one half and “2.5 mg” on the other half in black ink
5 mg: White opaque capsules imprinted “REV” on one half and “5 mg” on the other half in black ink
10 mg: Blue/green and pale yellow opaque capsules imprinted “REV” on one half and “10 mg” on the other half in bl
20 mg: Powder blue and blue-green opaque hard capsules imprinted “REV” on one half and “20 mg” on the other half in black ink25 mg: White opaque capsules imprinted “REV” on one half and “25 mg” on the other half in black ink
CONTRAINDICATIONS
Pregnancy
Lenalidomide can cause fetal harm when administered to a pregnant female. Limb abnormalities were seen in the offspring of monkeys that were dosed with lenalidomide during organogenesis. This effect was seen at all doses tested. Due to the results of this developmental monkey study, and lenalidomide’s structural similarities to thalidomide, a known human teratogen, lenalidomide is contraindicated in females who are pregnant. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus
Allergic Reactions
Lenalidomide is contraindicated in patients who have demonstrated hypersensitivity (e.g., angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis) to lenalidomide.
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity
Lenalidomide is a thalidomide analogue and is contraindicated for use during pregnancy. Thalidomide is a known human teratogen that causes life-threatening human birth defects or embryo-fetal death. An embryo-fetal development study in monkeys indicates that lenalidomide produced malformations in the offspring of female monkeys who received the drug during pregnancy, similar to birth defects observed in humans following exposure to thalidomide during pregnancy.
Lenalidomide is only available through the Lenalidomide REMS™ program.
Females of Reproductive Potential
Females of reproductive potential must avoid pregnancy for at least 4 weeks before beginning Lenalidomide therapy, during therapy, during dose interruptions and for at least 4 weeks after completing therapy.
Females must commit either to abstain continuously from heterosexual sexual intercourse or to use two methods of reliable birth control, beginning 4 weeks prior to initiating treatment with Lenalidomide, during therapy, during dose interruptions and continuing for 4 weeks following discontinuation of Lenalidomide therapy.
Two negative pregnancy tests must be obtained prior to initiating therapy. The first test should be performed within 10-14 days and the second test within 24 hours prior to prescribing Lenalidomide therapy and then weekly during the first month, then monthly thereafter in women with regular menstrual cycles or every 2 weeks in women with irregular menstrual cycles.
Males
Lenalidomide is present in the semen of patients receiving the drug. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking Lenalidomide and for up to 28 days after discontinuing Lenalidomide, even if they have undergone a successful vasectomy. Male patients taking Lenalidomide must not donate sperm.
Blood Donation
Patients must not donate blood during treatment with Lenalidomide and for 1 month following discontinuation of the drug because the blood might be given to a pregnant female patient whose fetus must not be exposed to Lenalidomide.
Hematologic Toxicity
Lenalidomide can cause significant neutropenia and thrombocytopenia. Patients taking Lenalidomide for MDS should have their complete blood counts monitored weekly for the first 8 weeks and at least monthly thereafter. Patients taking Lenalidomide for MM should have their complete blood counts monitored every 2 weeks for the first 12 weeks and then monthly thereafter. Patients taking Lenalidomide for MCL should have their complete blood counts monitored weekly for the first cycle (28 days), every 2 weeks during cycles 2-4, and then monthly thereafter. Patients may require dose interruption and/or dose reduction.
Grade 3 or 4 hematologic toxicity was seen in 80% of patients enrolled in the MDS study. In the 48% of patients who developed Grade 3 or 4 neutropenia, the median time to onset was 42 days (range, 14-411 days), and the median time to documented recovery was 17 days (range, 2-170 days). In the 54% of patients who developed Grade 3 or 4 thrombocytopenia, the median time to onset was 28 days (range, 8-290 days), and the median time to documented recovery was 22 days (range, 5-224 days.
In the pooled MM trials Grade 3 and 4 hematologic toxicities were more frequent in patients treated with the combination of Lenalidomide and dexamethasone than in patients treated with dexamethasone alone.
In the MCL trial, Grade 3 or 4 neutropenia was reported in 43% of the patients. Grade 3 or 4 thrombocytopenia was reported in 28% of the patients.
Venous Thromboembolism
Venous thromboembolic events (predominantly deep venous thrombosis and pulmonary embolism) have occurred in patients with multiple myeloma treated with lenalidomide combination therapy and patients with MDS or MCL treated with lenalidomide monotherapy. A significantly increased risk of DVT and PE was observed in patients with multiple myeloma who were treated with Lenalidomide and dexamethasone therapy in a clinical trial. It is not known whether prophylactic anticoagulation or antiplatelet therapy prescribed in conjunction with Lenalidomide may lessen the potential for venous thromboembolism. The decision to take prophylactic measures should be done carefully after an assessment of an individual patient’s underlying risk factors.
Increased Mortality in Patients with CLL
In a prospective randomized (1:1) clinical trial in the first line treatment of patients with chronic lymphocytic leukemia, single agent Lenalidomide therapy increased the risk of death as compared to single agent chlorambucil. In an interim analysis, there were 34 deaths among 210 patients on the Lenalidomide treatment arm compared to 18 deaths among 211 patients in the chlorambucil treatment arm, and hazard ratio for overall survival was 1.92 [95% CI: 1.08 – 3.41], consistent with a 92% increase in the risk of death. The trial was halted for safety in July 2013.
Serious adverse cardiovascular reactions, including atrial fibrillation, myocardial infarction, and cardiac failure occurred more frequently in the Lenalidomide treatment arm. Lenalidomide is not indicated and not recommended for use in CLL outside of controlled clinical trials.
Second Primary Malignancies
Patients with multiple myeloma treated with lenalidomide in studies including melphalan and stem cell transplantation had a higher incidence of second primary malignancies, particularly acute myelogenous leukemia (AML) and Hodgkin lymphoma, compared to patients in the control arms who received similar therapy but did not receive lenalidomide. Monitor patients for the development of second malignancies. Take into account both the potential benefit of lenalidomide and the risk of second primary malignancies when considering treatment with lenalidomide.
Hepatotoxicity
Hepatic failure, including fatal cases, has occurred in patients treated with lenalidomide in combination with dexamethasone. In clinical trials, 15% of patients experienced hepatotoxicity (with hepatocellular, cholestatic and mixed characteristics); 2% of patients with multiple myeloma and 1% of patients with myelodysplasia had serious hepatotoxicity events. The mechanism of drug-induced hepatotoxicity is unknown. Pre-existing viral liver disease, elevated baseline liver enzymes, and concomitant medications may be risk factors. Monitor liver enzymes periodically. Stop Lenalidomide upon elevation of liver enzymes. After return to baseline values, treatment at a lower dose may be considered.
Allergic Reactions
Angioedema and serious dermatologic reactions including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported. These events can be fatal. Patients with a prior history of Grade 4 rash associated with thalidomide treatment should not receive Lenalidomide. Lenalidomide interruption or discontinuation should be considered for Grade 2-3 skin rash. Lenalidomide must be discontinued for angioedema, Grade 4 rash, exfoliative or bullous rash, or if SJS or TEN is suspected and should not be resumed following discontinuation for these reactions.
Lenalidomide capsules contain lactose. Risk-benefit of Lenalidomide treatment should be evaluated in patients with lactose intolerance.
Tumor Lysis Syndrome
Fatal instances of tumor lysis syndrome have been reported during treatment with lenalidomide. The patients at risk of tumor lysis syndrome are those with high tumor burden prior to treatment. These patients should be monitored closely and appropriate precautions taken.
Tumor Flare Reaction
Tumor flare reaction has occurred during investigational use of lenalidomide for CLL and lymphoma, and is characterized by tender lymph node swelling, low grade fever, pain and rash. Lenalidomide is not indicated and not recommended for use in CLL outside of controlled clinical trials.
Monitoring and evaluation for tumor flare reaction (TFR) is recommended in patients with MCL. Tumor flare reaction may mimic progression of disease (PD). In the MCL trial, 13/134 (10%) of subjects experienced TFR; all reports were Grade 1 or 2 in severity. All of the events occurred in cycle 1 and one patient developed TFR again in cycle 11. Lenalidomide may be continued in patients with Grade 1 and 2 TFR without interruption or modification, at the physician’s discretion. Patients with Grade 1 and 2 TFR may also be treated with corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs) and/or narcotic analgesics for management of TFR symptoms. In patients with Grade 3 or 4 TFR, it is recommended to withhold treatment with lenalidomide until TFR resolves to = Grade 1. Patients with Grade 3 or 4 TFR may be treated for management of symptoms per the guidance for treatment of Grade 1 and 2 TFR.
ADVERSE REACTIONS
The following adverse reactions are described in detail in other labeling sections:
- Neutropenia and thrombocytopenia
- Deep vein thrombosis and pulmonary embolism
- Increased Mortality in Patients with CLL
- Second Primary Malignancies
- Hepatotoxicity
- Allergic Reactions
- Tumor lysis syndrome
- Tumor flare reactions
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Multiple Myeloma
Data were evaluated from 703 patients in two studies who received at least one dose of Lenalidomide/dexamethasone (353 patients) or placebo/dexamethasone (350 patients).
In the Lenalidomide/dexamethasone treatment group, 269 patients (76%) underwent at least one dose interruption with or without a dose reduction of Lenalidomide compared to 199 patients (57%) in the placebo/dexamethasone treatment group. Of these patients who had one dose interruption with or without a dose reduction, 50% in the Lenalidomide/dexamethasone treatment group underwent at least one additional dose interruption with or without a dose reduction compared to 21% in the placebo/dexamethasone treatment group. Most adverse events and Grade 3/4 adverse events were more frequent in patients who received the combination of Lenalidomide/dexamethasone compared to placebo/dexamethasone.
Tables 2, 3 and 4 summarize the adverse reactions reported for Lenalidomide/dexamethasone and placebo/dexamethasone groups.
| System Organ Class/ Preferred Term | Lenalidomide/Dex* (n=353) n (%) |
Placebo/Dex * (n=350) n (%) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Neutropenia % | 149 (42.2) | 22 (6.3) |
| Anemia @ | 111 (31.4) | 83 (23.7) |
| Thrombocytopenia @ | 76 (21.5) | 37 (10.6) |
| Leukopenia | 28 (7.9) | 4 (1.1) |
| Lymphopenia | 19 (5.4) | 5 (1.4) |
| General disorders and administration site conditions | ||
| Fatigue | 155 (43.9) | 146 (41.7) |
| Pyrexia | 97 (27.5) | 82 (23.4) |
| Peripheral edema | 93 (26.3) | 74 (21.1) |
| Chest Pain | 29 ( 8.2) | 20 (5.7) |
| Lethargy | 24 ( 6.8) | 8 (2.3) |
| Gastrointestinal disorders | ||
| Constipation | 143 (40.5) | 74 (21.1) |
| Diarrhea@ | 136 (38.5) | 96 (27.4) |
| Nausea @ | 92 (26.1) | 75 (21.4) |
| Vomiting @ | 43 (12.2) | 33 (9.4) |
| Abdominal Pain @ | 35 (9.9) | 22 (6.3) |
| Dry Mouth | 25 (7.1) | 13 (3.7) |
| Musculoskeletal and connective tissue disorders | ||
| Muscle cramp | 118 (33.4) | 74 (21.1) |
| Back pain | 91 (25.8) | 65 (18.6) |
| Bone Pain | 48 (13.6) | 39 (11.1) |
| Pain in Limb | 42 (11.9) | 32 (9.1) |
| Nervous system disorders | ||
| Dizziness | 82 (23.2) | 59 (16.9) |
| Tremor | 75 (21.2) | 26 (7.4) |
| Dysgeusia | 54 (15.3) | 34 (9.7) |
| Hypoaesthesia | 36 (10.2) | 25 (7.1) |
| Neuropathy a | 23 (6.5) | 13 (3.7) |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Dyspnea | 83 (23.5) | 60 (17.1) |
| Nasopharyngitis | 62 (17.6) | 31 (8.9) |
| Pharyngitis | 48 (13.6) | 33 (9.4) |
| Bronchitis | 40 (11.3) | 30 (8.6) |
| Infectionsb and infestations | ||
| Upper respiratory tract infection | 87 (24.6) | 55 (15.7) |
| Pneumonia @ | 48 (13.6) | 29 (8.3) |
| Urinary Tract Infection | 30 (8.5) | 19 (5.4) |
| Sinusitis | 26 (7.4) | 16 (4.6) |
| Skin and subcutaneous system disorders | ||
| Rash c | 75 (21.2) | 33 (9.4) |
| Sweating Increased | 35 (9.9) | 25 (7.1) |
| Dry Skin | 33 (9.3) | 14 (4.0) |
| Pruritus | 27 (7.6) | 18 (5.1) |
| Metabolism and nutrition disorders | ||
| Anorexia | 55 (15.6) | 34 (9.7) |
| Hypokalemia | 48 (13.6) | 21 (6.0) |
| Hypocalcemia | 31 (8.8) | 10 (2.9) |
| Appetite Decreased | 24 (6.8) | 14 (4.0) |
| Dehydration | 23 (6.5) | 15 (4.3) |
| Hypomagnesaemia | 24 (6.8) | 10 (2.9) |
| Investigations | ||
| Weight Decreased | 69 (19.5) | 52 (14.9) |
| Eye disorders | ||
| Blurred vision | 61 (17.3) | 40 (11.4) |
| Vascular disorders | ||
| Deep vein thrombosis % | 33 (9.3) | 15 (4.3) |
| Hypertension | 28 (7.9) | 20 (5.7) |
| Hypotension | 25 (7.1) | 15 (4.3) |
| System Organ Class/ Preferred Term | Lenalidomide/Dex# (n=353) n (%) |
Placebo/Dex# (n=350) n (%) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Neutropenia % | 118 (33.4) | 12 (3.4) |
| Thrombocytopenia @ | 43 (12.2) | 22 (6.3) |
| Anemia @ | 35 (9.9) | 20 (5.7) |
| Leukopenia | 14 (4.0) | 1 (0.3) |
| Lymphopenia | 10 (2.8) | 4 (1.1) |
| Febrile Neutropenia % | 8 (2.3) | 0 (0.0) |
| General disorders and administration site conditions | ||
| Fatigue | 23 (6.5) | 17 (4.9) |
| Vascular disorders | ||
| Deep vein thrombosis % | 29 (8.2) | 12 (3.4) |
| Infectionsb and infestations | ||
| Pneumonia @ | 30 (8.5) | 19 (5.4) |
| Urinary Tract Infection | 5 (1.4) | 1 (0.3) |
| Metabolism and nutrition disorders | ||
| Hypokalemia | 17 (4.8) | 5 (1.4) |
| Hypocalcemia | 13 (3.7) | 6 (1.7) |
| Hypophosphatemia | 9 (2.5) | 0 (0.0) |
| Respiratory, thoracic and mediastinal disorders | ||
| Pulmonary embolism@ | 14 (4.0) | 3 (0.9) |
| Respiratory Distress @ | 4 (1.1) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | ||
| Muscle weakness | 20 (5.7) | 10 (2.9) |
| Gastrointestinal disorders | ||
| Diarrhea @ | 11 (3.1) | 4 (1.1) |
| Constipation | 7 (2.0) | 1 (0.3) |
| Nausea @ | 6 (1.7) | 2 (0.6) |
| Cardiac disorders | ||
| Atrial fibrillation @ | 13 (3.7) | 4 (1.1) |
| Tachycardia | 6 (1.7) | 1 (0.3) |
| Cardiac Failure Congestive @ | 5 (1.4) | 1 (0.3) |
| Nervous System disorders | ||
| Syncope | 10 (2.8) | 3 (0.9) |
| Dizziness | 7 (2.0) | 3 (0.9) |
| Eye Disorders | ||
| Cataract | 6 (1.7) | 1 (0.3) |
| Cataract Unilateral | 5 (1.4) | 0 (0.0) |
| Psychiatric Disorder | ||
| Depression | 10 (2.8) | 6 (1.7) |
|
For all tables above: n – Number of Patients * - All Treatment Emergent AEs with =5% of Patients in Lenalidomide/ Dex and at Least 2% Difference in Proportion between the Two Arms - (Safety population) # - All Treatment Emergent Grades 3 and 4 AEs with =1% Patients in Lenalidomide/ Dex and at Least 1% Difference in Proportion between the Two Arms - (Safety population) & - All Treatment Emergent Serious AEs with =1% Patients in Lenalidomide/ Dex and at Least 1% Difference in Proportion between the Two Arms - (Safety population) @ - ADRs with Death as an outcome % - ADRs which were considered to be life threatening (if the outcome of the event was death, it is included with death cases) a - All PTs under the MedDRA SMQ of Neuropathy of a peripheral sensory nature will be considered listed b - All PTs under SOC of Infections except for rare infections of Public Health interest will be considered listed c - All PTs under HLT of Rash will be considered listed Dex=dexamethasone Median duration of exposure among patients treated with Lenalidomide/dexamethasone was 44 weeks while median duration of exposure among patients treated with placebo/dexamethasone was 23 weeks. This should be taken into consideration when comparing frequency of adverse events between two treatment groups Lenalidomide/dexamethasone vs. placebo/dexamethasone. |
||
| System Organ Class/ Preferred Term | Lenalidomide/Dex& (n=353) n (%) |
Placebo/Dex& (n=350) n (%) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Febrile Neutropenia% | 6 (1.7) | 0 (0.0) |
| Vascular disorders | ||
| Deep vein thrombosis% | 26 (7.4) | 11 (3.1) |
| Infectionsb and infestations | ||
| Pneumonia @ | 33 (9.3) | 21 (6.0) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Pulmonary embolism@ | 13 (3.7) | 3 (0.9) |
| Cardiac disorders | ||
| Atrial fibrillation @ | 11 (3.1) | 2 (0.6) |
| Cardiac Failure Congestive @ | 5 (1.4) | 0 (0.0) |
| Nervous system disorders | ||
| Cerebrovascular accident @ | 7 (2.0) | 3 (0.9) |
| Gastrointestinal disorders | ||
| Diarrhea @ | 6 (1.7) | 2 (0.6) |
| Musculoskeletal and connective tissue disorders | ||
| Bone Pain | 4 (1.1) | 0 (0.0) |
Venous Thromboembolism
Deep Vein Thrombosis and Pulmonary Embolism [see Warnings and Precautions]
Deep vein thrombosis (DVT) was reported as a serious adverse drug reaction (7.4%) or Grade 3/4 (8.2%) at a higher rate in the Lenalidomide/dexamethasone group compared to 3.1 % and 3.4% in the placebo/dexamethasone group, respectively. Discontinuations due to DVT adverse reactions were reported at comparable rates between groups.
Pulmonary embolism (PE) was reported as a serious adverse drug reaction including Grade 3/4 (3.7%) at a higher rate in the Lenalidomide/dexamethasone group compared to 0.9% in the placebo/dexamethasone group. Discontinuations due to PE adverse reactions were reported at comparable rates between groups.
Other Adverse Reactions
In these clinical studies of Lenalidomide in patients with multiple myeloma, the following adverse drug reactions (ADRs) not described above that occurred at =1% rate and of at least twice of the placebo percentage rate were reported:
Blood and lymphatic system disorders:
pancytopenia, autoimmune hemolytic anemia
Cardiac disorders:
bradycardia, myocardial infarction, angina pectoris
Endocrine disorders:
hirsutism
Eye disorders:
blindness, ocular hypertension
Gastrointestinal disorders:
gastrointestinal hemorrhage, glossodynia
General disorders and administration site conditions:
malaise
Investigations:
liver function tests abnormal, alanine aminotransferase increased
Nervous system disorders:
cerebral ischemia
Psychiatric disorders:
mood swings, hallucination, loss of libido
Reproductive system and breast disorders:
erectile dysfunction
Respiratory, thoracic and mediastinal disorders:
cough, hoarseness
Skin and subcutaneous tissue disorders:
exanthem, skin hyperpigmentation
Clinical Trials Experience in Myelodysplastic Syndromes
A total of 148 patients received at least 1 dose of 10 mg Lenalidomide in the del 5q MDS clinical study. At least one adverse event was reported in all of the 148 patients who were treated with the 10 mg starting dose of Lenalidomide. The most frequently reported adverse events were related to blood and lymphatic system disorders, skin and subcutaneous tissue disorders, gastrointestinal disorders, and general disorders and administrative site conditions.
Thrombocytopenia (61.5%; 91/148) and neutropenia (58.8%; 87/148) were the most frequently reported adverse events. The next most common adverse events observed were diarrhea (48.6%; 72/148), pruritus (41.9%; 62/148), rash (35.8%; 53/148) and fatigue (31.1%; 46/148). Table 5 summarizes the adverse events that were reported in = 5% of the Lenalidomide treated patients in the del 5q MDS clinical study. Table 6 summarizes the most frequently observed Grade 3 and Grade 4 adverse reactions regardless of relationship to treatment with Lenalidomide. In the single-arm studies conducted, it is often not possible to distinguish adverse events that are drug-related and those that reflect the patient’s underlying disease.
|
[a] System organ classes and preferred terms are coded using the MedDRA dictionary. System organ classes and preferred terms are listed in descending order of frequency for the Overall column. A patient with multiple occurrences of an AE is counted only once in the AE category. |
|
| System organ class/Preferred term [a] | 10 mg Overall (N=148) |
|---|---|
| Patients with at least one adverse event | 148 (100.0) |
| Blood and Lymphatic System Disorders Thrombocytopenia Neutropenia Anemia Leukopenia Febrile Neutropenia | 91 (61.5) 87 (58.8) 17 (11.5) 12 (8.1) 8 (5.4) |
| Skin and Subcutaneous Tissue Disorders Pruritus Rash Dry Skin Contusion Night Sweats Sweating Increased Ecchymosis Erythema | 62 (41.9) 53 (35.8) 21 (14.2) 12 (8.1) 12 (8.1) 10 (6.8) 8 (5.4) 8 (5.4) |
| Gastrointestinal Disorders Diarrhea Constipation Nausea Abdominal Pain Vomiting Abdominal Pain Upper Dry Mouth Loose Stools | 72 (48.6) 35 (23.6) 35 (23.6) 18 (12.2) 15 (10.1) 12 (8.1) 10 (6.8) 9 (6.1) |
| Respiratory, Thoracic and Mediastinal Disorders Nasopharyngitis Cough Dyspnea Pharyngitis Epistaxis Dyspnea Exertional Rhinitis Bronchitis | 34 (23.0) 29 (19.6) 25 (16.9) 23 (15.5) 22 (14.9) 10 (6.8) 10 (6.8) 9 (6.1) |
| General Disorders and Administration Site Conditions Fatigue Pyrexia Edema Peripheral Asthenia Edema Pain Rigors Chest Pain | 46 (31.1) 31 (20.9) 30 (20.3) 22 (14.9) 15 (10.1) 10 (6.8) 9 (6.1) 8 (5.4) |
| Musculoskeletal and Connective Tissue Disorders Arthralgia Back Pain Muscle Cramp Pain in Limb Myalgia Peripheral Swelling | 32 (21.6) 31 (20.9) 27 (18.2) 16 (10.8) 13 (8.8) 12 (8.1) |
| Nervous System Disorders Dizziness Headache Hypoesthesia Dysgeusia Peripheral Neuropathy | 29 (19.6) 29 (19.6) 10 (6.8) 9 (6.1) 8 (5.4) |
| Infections and Infestations Upper Respiratory Tract Infection Pneumonia Urinary Tract Infection Sinusitis Cellulitis | 22 (14.9) 17 (11.5) 16 (10.8) 12 (8.1) 8 (5.4) |
| Metabolism and Nutrition Disorders Hypokalemia Anorexia Hypomagnesemia | 16 (10.8) 15 (10.1) 9 (6.1) |
| Investigations Alanine Aminotransferase Increased | 12 (8.1) |
| Psychiatric Disorders Insomnia Depression | 15 (10.1) 8 (5.4) |
| Renal and Urinary Disorders Dysuria | 10 (6.8) |
| Vascular Disorders Hypertension | 9 ( 6.1) |
| Endocrine Disorders Acquired Hypothyroidism | 10 (6.8) |
| Cardiac Disorders Palpitations | 8 (5.4) |
|
[1] Adverse events with frequency =1% in the 10 mg Overall group. Grade 3 and 4 are based on National Cancer Institute Common Toxicity Criteria version 2. [2] Preferred Terms are coded using the MedDRA dictionary. A patient with multiple occurrences of an AE is counted only once in the Preferred Term category. |
|
| Preferred term [2] | 10 mg (N=148) |
|---|---|
| Patients with at least one Grade 3/4 AE | 131 (88.5) |
| Neutropenia | 79 (53.4) |
| Thrombocytopenia | 74 (50.0) |
| Pneumonia | 11 (7.4) |
| Rash | 10 (6.8) |
| Anemia | 9 (6.1) |
| Leukopenia | 8 (5.4) |
| Fatigue | 7 (4.7) |
| Dyspnea | 7 (4.7) |
| Back Pain | 7 (4.7) |
| Febrile Neutropenia | 6 (4.1) |
| Nausea | 6 (4.1) |
| Diarrhea | 5 (3.4) |
| Pyrexia | 5 (3.4) |
| Sepsis | 4 (2.7) |
| Dizziness | 4 (2.7) |
| Granulocytopenia | 3 (2.0) |
| Chest Pain | 3 (2.0) |
| Pulmonary Embolism | 3 (2.0) |
| Respiratory Distress | 3 (2.0) |
| Pruritus | 3 (2.0) |
| Pancytopenia | 3 (2.0) |
| Muscle Cramp | 3 (2.0) |
| Respiratory Tract Infection | 2 (1.4) |
| Upper Respiratory Tract Infection | 2 (1.4) |
| Asthenia | 2 (1.4) |
| Multi-organ Failure | 2 (1.4) |
| Epistaxis | 2 (1.4) |
| Hypoxia | 2 (1.4) |
| Pleural Effusion | 2 (1.4) |
| Pneumonitis | 2 (1.4) |
| Pulmonary Hypertension | 2 (1.4) |
| Vomiting | 2 (1.4) |
| Sweating Increased | 2 (1.4) |
| Arthralgia | 2 (1.4) |
| Pain in Limb | 2 (1.4) |
| Headache | 2 (1.4) |
| Syncope | 2 (1.4) |
In other clinical studies of Lenalidomide in MDS patients, the following serious adverse events (regardless of relationship to study drug treatment) not described in Table 5 or 6 were reported:
Blood and lymphatic system disorders:
warm type hemolytic anemia, splenic infarction, bone marrow depression, coagulopathy, hemolysis, hemolytic anemia, refractory anemia
Cardiac disorders:
cardiac failure congestive, atrial fibrillation, angina pectoris, cardiac arrest, cardiac failure, cardio-respiratory arrest, cardiomyopathy, myocardial infarction, myocardial ischemia, atrial fibrillation aggravated, bradycardia, cardiogenic shock, pulmonary edema, supraventricular arrhythmia, tachyarrhythmia, ventricular dysfunction
Ear and labyrinth disorders:
vertigo
Endocrine disorders:
Basedow’s disease
Gastrointestinal disorders:
gastrointestinal hemorrhage, colitis ischemic, intestinal perforation, rectal hemorrhage, colonic polyp, diverticulitis, dysphagia, gastritis, gastroenteritis, gastroesophageal reflux disease, obstructive inguinal hernia, irritable bowel syndrome, melena, pancreatitis due to biliary obstruction, pancreatitis, perirectal abscess, small intestinal obstruction, upper gastrointestinal hemorrhage
General disorders and administration site conditions:
disease progression, fall, gait abnormal, intermittent pyrexia, nodule, rigors, sudden death
Hepatobiliary disorders:
hyperbilirubinemia, cholecystitis, acute cholecystitis, hepatic failure
Immune system disorders:
hypersensitivity
Infections and infestations
infection bacteremia, central line infection, clostridial infection, ear infection, Enterobacter sepsis, fungal infection, herpes viral infection NOS, influenza, kidney infection, Klebsiella sepsis, lobar pneumonia, localized infection, oral infection, Pseudomonas infection, septic shock, sinusitis acute, sinusitis, Staphylococcal infection, urosepsis
Injury, poisoning and procedural complications:
femur fracture, transfusion reaction, cervical vertebral fracture, femoral neck fracture, fractured pelvis, hip fracture, overdose, post procedural hemorrhage, rib fracture, road traffic accident, spinal compression fracture
Investigations:
blood creatinine increased, hemoglobin decreased, liver function tests abnormal, troponin I increased
Metabolism and nutrition disorders:
dehydration, gout, hypernatremia, hypoglycemia
Musculoskeletal and connective tissue disorders:
arthritis, arthritis aggravated, gouty arthritis, neck pain, chondrocalcinosis pyrophosphate
Neoplasms benign, malignant and unspecified:
acute leukemia, acute myeloid leukemia, bronchoalveolar carcinoma, lung cancer metastatic, lymphoma, prostate cancer metastatic
Nervous system disorders:
cerebrovascular accident, aphasia, cerebellar infarction, cerebral infarction, depressed level of consciousness, dysarthria, migraine, spinal cord compression, subarachnoid hemorrhage, transient ischemic attack
Psychiatric disorders:
confusional state
Renal and urinary disorders:renal failure, hematuria, renal failure acute, azotemia, calculus ureteric, renal mass
Reproductive system and breast disorders:
pelvic pain
Respiratory, thoracic and mediastinal disorders:
bronchitis, chronic obstructive airways disease exacerbated, respiratory failure, dyspnea exacerbated, interstitial lung disease, lung infiltration, wheezing
Skin and subcutaneous tissue disorders:
acute febrile neutrophilic dermatosis
Vascular system disorders:
deep vein thrombosis, hypotension, aortic disorder, ischemia, thrombophlebitis superficial, thrombosis
Clinical Trials Experience in Mantle Cell Lymphoma
In the MCL trial, a total of 134 patients received at least 1 dose of Lenalidomide. Their median age was 67 (range 43-83) years, 128/134 (96%) were Caucasian, 108/134 (81%) were males and 82/134 (61%) had duration of MCL for at least 3 years.
Table 7 summarizes the most frequently observed adverse reactions regardless of relationship to treatment with Lenalidomide. Across the 134 patients treated in this study, median duration of treatment was 95 days (1-1002 days). Seventy-eight patients (58%) received 3 or more cycles of therapy, 53 patients (40%) received 6 or more cycles, and 26 patients (19%) received 12 or more cycles. Seventy-six patients (57%) underwent at least one dose interruption due to adverse events, and 51 patients (38%) underwent at least one dose reduction due to adverse events. Twenty-six patients (19%) discontinued treatment due to adverse events.
|
1-MCL trial AEs – All treatment emergent AEs with =10% of subjects 2-MCL trial Grade 3/4 AEs – All treatment-emergent Grade 3/4 AEs in 2 or more subjects $-MCL trial Serious AEs – All treatment-emergent SAEs in 2 or more subjects @ - AEs where at least one resulted in a fatal outcome % - AEs where at least one was considered to be Life Threatening (if the outcome of the event was death, it is included with death cases) #- All PTs under SOC of Infections except for rare infections of Public Health interest will be considered listed +- All PTs under HLT of Rash will be considered listed |
||
| System Organ Class/Preferred Term |
All AEs1 (N=134) n (%) |
Grade 3/4 AEs2 (N=134) n (%) |
|---|---|---|
| General disorders and administration site conditions | ||
| Fatigue | 45 (34) | 9 (7) |
| Pyrexia$ | 31 (23) | 3 (2) |
| Edema peripheral | 21 (16) | 0 |
| Asthenia$ | 19 (14) | 4 (3) |
| General physical health deterioration | 3 (2) | 2 (1) |
| Gastrointestinal disorders | ||
| Diarrhea$ | 42 (31) | 8 (6) |
| Nausea$ | 40 (30) | 1 (<1) |
| Constipation | 21 (16) | 1 (<1) |
| Vomiting$ | 16 (12) | 1 (<1) |
| Abdominal pain$ | 13 (10) | 5 (4) |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 18 (13) | 2 (1) |
| Muscle spasms | 17 (13) | 1 (<1) |
| Arthralgia | 11 (8) | 2 (1) |
| Muscular weakness$ | 8 (6) | 2 (1) |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | 38 (28) | 1 (<1) |
| Dyspnea$ | 24 (18) | 8 (6) |
| Pleural Effusion | 10 (7) | 2 (1) |
| Hypoxia | 3 (2) | 2 (1) |
| Pulmonary embolism | 3 (2) | 2 (1) |
| Respiratory distress$ | 2 (1) | 2 (1) |
| Oropharyngeal pain | 13 (10) | 0 |
| Infections and infestations | ||
| Pneumonia@$ | 19 (14) | 12 (9) |
| Upper respiratory tract infection | 17 (13) | 0 |
| Cellulitis$ | 3 (2) | 2 (1) |
| Bacteremia$ | 2 (1) | 2 (1) |
| Staphylococcal sepsis$ | 2 (1) | 2 (1) |
| Urinary tract infection$ | 5 (4) | 2 (1) |
| Skin and subcutaneous tissue disorders | ||
| Rash+ | 30 (22) | 2 (1) |
| Pruritus | 23 (17) | 1 (<1) |
| Blood and lymphatic system disorders | ||
| Neutropenia | 65 (49) | 58 (43) |
| Thrombocytopenia%$ | 48 (36) | 37 (28) |
| Anemia$ | 41 (31) | 15 (11) |
| Leukopenia$ | 20 (15) | 9 (7) |
| Lymphopenia | 10 (7) | 5 (4) |
| Febrile neutropenia$ | 8 (6) | 8 (6) |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 19 (14) | 1 (<1) |
| Hypokalemia | 17 (13) | 3 (2) |
| Dehydration$ | 10 (7) | 4 (3) |
| Hypocalcemia | 4 (3) | 2 (1) |
| Hyponatremia | 3 (2) | 3 (2) |
| Renal and urinary disorders | ||
| Renal failure$ | 5 (4) | 2 (1) |
| Vascular disorders | ||
| Hypotension@$ | 9 (7) | 4 (3) |
| Deep vein thrombosis$ | 5 (4) | 5 (4) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | ||
| Tumor flare | 13 (10) | 0 |
| Squamous cell carcinoma of skin$ | 4 (3) | 4 (3) |
| Investigations | ||
| Weight decreased | 17 (13) | 0 |
The following adverse events which have occurred in other indications and not described above have been reported (5-10%) in patients treated with Lenalidomide monotherapy for mantle cell lymphoma.
General disorders and administration site conditions: Chills
Musculoskeletal and connective tissue disorders: Pain in extremity
Nervous system disorders: Dysguesia, headache, neuropathy peripheral
Infections and infestations: Respiratory tract infection, sinusitis, nasopharyngitis
Skin and subcutaneous tissue disorders: Dry skin, night sweats
The following serious adverse events not described above and reported in 2 or more patients treated with Lenalidomide monotherapy for mantle cell lymphoma.
Respiratory, Thoracic and Mediastinal Disorders: Chronic obstructive pulmonary disease
Infections and Infestations: Clostridium difficile colitis, sepsis
Neoplasms benign, malignant and unspecified (incl cysts and polyps): Basal cell carcinoma
Cardiac Disorder: Supraventricular tachycardia
Postmarketing Experience
The following adverse drug reactions have been identified from the worldwide post-marketing experience with Lenalidomide: Allergic conditions (angioedema, SJS, TEN), tumor lysis syndrome (TLS) and tumor flare reaction (TFR), pneumonitis, hepatic failure, including fatality, toxic hepatitis, cytolytic hepatitis, cholestatic hepatitis, and mixed cytolytic/cholestatic hepatitis and transient abnormal liver laboratory tests. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cases of hypothyroidism and hyperthyroidism have also been reported. Optimal control of thyroid function is recommended before start of treatment. Baseline and ongoing monitoring of thyroid function is recommended.
DRUG INTERACTIONS
Results from human in vitro studies show that Lenalidomide is neither metabolized by nor inhibits or induces the cytochrome P450 pathway suggesting that lenalidomide is not likely to cause or be subject to P450-based metabolic drug interactions.
In vitro studies demonstrated that Lenalidomide is not a substrate of human breast cancer resistance protein (BCRP), multidrug resistance protein (MRP) transporters MRP1, MRP2, or MRP3, organic anion transporters (OAT) OAT1 and OAT3, organic anion transporting polypeptide 1B1 (OATP1B1 or OATP2), organic cation transporters (OCT) OCT1 and OCT2, multidrug and toxin extrusion protein (MATE) MATE1, and organic cation transporters novel (OCTN) OCTN1 and OCTN2.
In vitro, lenalidomide is a substrate, but is not an inhibitor of P-glycoprotein (P-gp).
Digoxin
When digoxin was co-administered with multiple doses of Lenalidomide (10 mg/day) the digoxin Cmax and AUC0-8 were increased by 14%. Periodic monitoring of digoxin plasma levels, in accordance with clinical judgment and based on standard clinical practice in patients receiving this medication, is recommended during administration of Lenalidomide.
Warfarin
Co-administration of multiple dose Lenalidomide (10 mg) with single dose warfarin (25 mg) had no effect on the pharmacokinetics of total lenalidomide or R- and S-warfarin. Expected changes in laboratory assessments of PT and INR were observed after warfarin administration, but these changes were not affected by concomitant Lenalidomide administration. It is not known whether there is an interaction between dexamethasone and warfarin. Close monitoring of PT and INR is recommended in multiple myeloma patients taking concomitant warfarin.
Concomitant Therapies That May Increase the Risk of Thrombosis
Erythropoietic agents, or other agents that may increase the risk of thrombosis, such as estrogen containing therapies, should be used with caution in multiple myeloma patients receiving lenalidomide with dexamethasone.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Category X [see Boxed Warnings and Contraindications]
Risk Summary
Lenalidomide can cause embryo-fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Lenalidomide is a thalidomide analogue.
Thalidomide is a human teratogen, inducing a high frequency of severe and life-threatening birth defects such as amelia (absence of limbs), phocomelia (short limbs), hypoplasticity of the bones, absence of bones, external ear abnormalities (including anotia, micropinna, small or absent external auditory canals), facial palsy, eye abnormalities (anophthalmos, microphthalmos), and congenital heart defects. Alimentary tract, urinary tract, and genital malformations have also been documented and mortality at or shortly after birth has been reported in about 40% of infants.
Lenalidomide caused thalidomide-type limb defects in monkey offspring. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
If pregnancy does occur during treatment, immediately discontinue the drug. Under these conditions, refer patient to an obstetrician/gynecologist experienced in reproductive toxicity for further evaluation and counseling. Any suspected fetal exposure to Lenalidomide must be reported to the FDA via the MedWatch program at 1-800-332-1088 and also to Celgene Corporation at 1-888-423-5436.
Animal data
In an embryo-fetal developmental toxicity study in monkeys, teratogenicity, including thalidomide-like limb defects, occurred in offspring when pregnant monkeys received oral lenalidomide during organogenesis. Exposure (AUC) in monkeys at the lowest dose was 0.17 times the human exposure at the maximum recommended human dose (MRHD) of 25 mg. Similar studies in pregnant rabbits and rats at 20 times and 200 times the MRHD respectively, produced embryo lethality in rabbits and no adverse reproductive effects in rats.
In a pre- and post-natal development study in rats, animals received lenalidomide from organogenesis through lactation. The study revealed a few adverse effects on the offspring of female rats treated with lenalidomide at doses up to 500 mg/kg (approximately 200 times the human dose of 25 mg based on body surface area). The male offspring exhibited slightly delayed sexual maturation and the female offspring had slightly lower body weight gains during gestation when bred to male offspring. As with thalidomide, the rat model may not adequately address the full spectrum of potential human embryo-fetal developmental effects for lenalidomide.
Nursing mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from lenalidomide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric use
Safety and effectiveness in pediatric patients below the age of 18 have not been established.
Geriatric use
Lenalidomide has been used in multiple myeloma (MM) clinical trials in patients up to 86 years of age.
Of the 703 MM patients who received study treatment in Studies 1 and 2, 45% were age 65 or over while 12% of patients were age 75 and over. The percentage of patients age 65 or over was not significantly different between the Lenalidomide/dexamethasone and placebo/dexamethasone groups. Of the 353 patients who received Lenalidomide/dexamethasone, 46% were age 65 and over. In both studies, patients > 65 years of age were more likely than patients = 65 years of age to experience DVT, pulmonary embolism, atrial fibrillation, and renal failure following use of Lenalidomide. No differences in efficacy were observed between patients over 65 years of age and younger patients.
Lenalidomide has been used in del 5q MDS clinical trials in patients up to 95 years of age.
Of the 148 patients with del 5q MDS enrolled in the major study, 38% were age 65 and over, while 33% were age 75 and over. Although the overall frequency of adverse events (100%) was the same in patients over 65 years of age as in younger patients, the frequency of serious adverse events was higher in patients over 65 years of age than in younger patients (54% vs. 33%). A greater proportion of patients over 65 years of age discontinued from the clinical studies because of adverse events than the proportion of younger patients (27% vs.16%). No differences in efficacy were observed between patients over 65 years of age and younger patients.
Lenalidomide has been used in a mantle cell lymphoma (MCL) clinical trial in patients up to 83 years of age. Of the 134 patients with MCL enrolled in the MCL trial, 63% were age 65 and over, while 22% of patients were age 75 and over. The overall frequency of adverse events was similar in patients over 65 years of age and in younger patients (98% vs. 100%). The overall incidence of grade 3 and 4 adverse events was also similar in these 2 patient groups (79% vs. 78%, respectively). The frequency of serious adverse events was higher in patients over 65 years of age than in younger patients (55% vs. 41%). No differences in efficacy were observed between patients over 65 years of age and younger patients.
Since elderly patients are more likely to have decreased renal function, care should be taken in dose selection. Monitor renal function.
Females of Reproductive Potential and Males
Lenalidomide can cause fetal harm when administered during pregnancy. Females of reproductive potential must avoid pregnancy 4 weeks before therapy, while taking Lenalidomide, during dose interruptions and for at least 4 weeks after completing therapy.
Females
Females of reproductive potential must commit either to abstain continuously from heterosexual sexual intercourse or to use two methods of reliable birth control simultaneously (one highly effective form of contraception – tubal ligation, IUD, hormonal (birth control pills, injections, hormonal patches, vaginal rings or implants) or partner’s vasectomy and one additional effective contraceptive method – male latex or synthetic condom, diaphragm or cervical cap. Contraception must begin 4 weeks prior to initiating treatment with Lenalidomide, during therapy, during dose interruptions and continuing for 4 weeks following discontinuation of Lenalidomide therapy. Reliable contraception is indicated even where there has been a history of infertility, unless due to hysterectomy. Females of reproductive potential should be referred to a qualified provider of contraceptive methods, if needed.
Females of reproductive potential must have 2 negative pregnancy tests before initiating Lenalidomide. The first test should be performed within 10-14 days, and the second test within 24 hours prior to prescribing Lenalidomide. Once treatment has started and during dose interruptions, pregnancy testing for females of reproductive potential should occur weekly during the first 4 weeks of use, then pregnancy testing should be repeated every 4 weeks in females with regular menstrual cycles. If menstrual cycles are irregular, the pregnancy testing should occur every 2 weeks. Pregnancy testing and counseling should be performed if a patient misses her period or if there is any abnormality in her menstrual bleeding. Lenalidomide treatment must be discontinued during this evaluation.
Males
Lenalidomide is present in the semen of males who take Lenalidomide. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking Lenalidomide, during dose interruptions and for up to 28 days after discontinuing Lenalidomide, even if they have undergone a successful vasectomy. Male patients taking Lenalidomide must not donate sperm
Renal Impairment
Since lenalidomide is primarily excreted unchanged by the kidney, adjustments to the starting dose of Lenalidomide are recommended to provide appropriate drug exposure in patients with moderate (CLcr 30-60 mL/min) or severe renal impairment (CLcr < 30 mL/min) and in patients on dialysis.
Hepatic Impairment
No dedicated study has been conducted in patients with hepatic impairment. The elimination of unchanged lenalidomide is predominantly by the renal route.
OVERDOSAGE
There is no specific experience in the management of lenalidomide overdose in patients; although in dose-ranging studies, some patients were exposed to up to 150 mg and in single-dose studies, some patients were exposed to up to 400 mg.
In studies, the dose-limiting toxicity was essentially hematological. In the event of overdose, supportive care is advised.
DESCRIPTION
Lenalidomide, a thalidomide analogue, is an immunomodulatory agent with antiangiogenic and antineoplastic properties. The chemical name is 3-(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione and it has the following chemical structure:

3-(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione
The empirical formula for lenalidomide is C13H13N3O3, and the gram molecular weight is 259.3.
Lenalidomide is an off-white to pale-yellow solid powder. It is soluble in organic solvent/water mixtures, and buffered aqueous solvents. Lenalidomide is more soluble in organic solvents and low pH solutions. Solubility was significantly lower in less acidic buffers, ranging from about 0.4 to 0.5 mg/ml. Lenalidomide has an asymmetric carbon atom and can exist as the optically active forms S(-) and R(+), and is produced as a racemic mixture with a net optical rotation of zero.
Lenalidomide is available in 2.5 mg, 5 mg, 10 mg, 15 mg, 20 mg and 25 mg capsules for oral administration. Each capsule contains lenalidomide as the active ingredient and the following inactive ingredients: lactose anhydrous, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The 5 mg and 25 mg capsule shell contains gelatin, titanium dioxide and black ink. The 2.5 mg and 10 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink. The 15 mg capsule shell contains gelatin, FD&C blue #2, titanium dioxide and black ink. The 20 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink.
12 CLINICAL PHARMACOLOGY
Mechanism of action
Lenalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Lenalidomide inhibits proliferation and induces apoptosis of certain hematopoietic tumor cells including multiple myeloma, mantle cell lymphoma, and del (5q) myelodysplastic syndromes in vitro. Lenalidomide causes a delay in tumor growth in some in vivo nonclinical hematopoietic tumor models including multiple myeloma. Immunomodulatory properties of lenalidomide include activation of T cells and natural killer (NK) cells, increased numbers of NKT cells, and inhibition of pro-inflammatory cytokines (e.g., TNF-a and IL-6) by monocytes. In multiple myeloma cells, the combination of lenalidomide and dexamethasone synergizes the inhibition of cell proliferation and the induction of apoptosis.
Pharmacodynamics
The effect of lenalidomide on the QTc interval was evaluated in 60 healthy male subjects in a randomized, thorough QT study with placebo and positive controls. At a dose two times the maximum recommended dose, lenalidomide does not prolong the QTc interval to any clinically relevant extent. The largest upper bound of the 2-sided 90% CI for the mean differences between lenalidomide and placebo was below 10 ms.
Pharmacokinetics
Absorption
Lenalidomide is rapidly absorbed following oral administration. Following single and multiple doses of Lenalidomide in patients with MM or MDS the maximum plasma concentrations occurred between 0.5 and 6 hours post-dose. The single and multiple dose pharmacokinetic disposition of lenalidomide is linear with AUC and Cmax values increasing proportionally with dose. Multiple dosing at the recommended dose-regimen does not result in drug accumulation.
Systemic exposure (AUC) of lenalidomide in MM and MDS patients with normal or mild renal function (CLcr = 60 mL/min) is approximately 60% higher as compared to young healthy male subjects.
Administration of a single 25 mg dose of Lenalidomide with a high-fat meal in healthy subjects reduces the extent of absorption, with an approximate 20% decrease in AUC and 50% decrease in Cmax. In the trials where the efficacy and safety were established for Lenalidomide, the drug was administered without regard to food intake. Lenalidomide can be administered with or without food.
Population pharmacokinetic analyses show that the oral absorption rate of lenalidomide in patients with MCL is similar to that observed in patients with MM or MDS.
Distribution
In vitro (14C)-lenalidomide binding to plasma proteins is approximately 30%.
Metabolism
Lenalidomide -undergoes limited metabolism. Unchanged lenalidomide is the predominant circulating component in humans. Two identified metabolites are hydroxy-lenalidomide and N-acetyl-lenalidomide; each constitutes less than 5% of parent levels in circulation.
Elimination
Elimination is primarily renal. Following a single oral administration of [14C]-lenalidomide (25 mg) to healthy subjects, approximately 90% and 4% of the radioactive dose is eliminated within ten days in urine and feces, respectively. Approximately 82% of the radioactive dose is excreted as lenalidomide in the urine within 24 hours. Hydroxy-lenalidomide and N-acetyl-lenalidomide represent 4.59% and 1.83% of the excreted dose, respectively. The renal clearance of lenalidomide exceeds the glomerular filtration rate.
The mean half-life of lenalidomide is 3 hours in healthy subjects and 3 to 5 hours in patients with MM, MDS or MCL.
Effect of Dexamethasone
Co-administration of single or multiple doses of dexamethasone (40 mg) has no clinically relevant effect on the multiple dose pharmacokinetics of Lenalidomide (25 mg).
Specific Populations
Patients with Renal Impairment:
The pharmacokinetics of lenalidomide were studied in patients with renal impairment due to nonmalignant conditions. In this study, 5 patients with mild renal impairment (creatinine clearance 57-74 mL/min), 6 patients with moderate renal impairment (creatinine clearance 33-46 mL/min), 6 patients with severe renal impairment (creatinine clearance 17-29 mL/min), and 6 patients with end stage renal disease requiring dialysis were administered a single oral 25-mg dose of Lenalidomide. As a control group comparator, 7 healthy subjects of similar age with normal renal function (creatinine clearance 83-145 mL/min) were also administered a single oral 25-mg dose of Lenalidomide. As creatinine clearance decreased from mild to severe impairment, half-life increased and drug clearance decreased linearly. Patients with moderate and severe renal impairment had a 3-fold increase in half-life and a 66% to 75% decrease in drug clearance compared to healthy subjects. Patients on hemodialysis (n=6) given a single, 25-mg dose of lenalidomide has an approximate 4.5-fold increase in half-life and an 80% decrease in drug clearance compared to healthy subjects. Approximately 40% of the administered dose was removed from the body during a single dialysis session.
In MM patients, those patients with mild renal impairment had an AUC 56% greater than those with normal renal function.
Adjustment of the starting dose of Lenalidomide is recommended in patients with moderate or severe (CLcr < 60 mL/min) renal impairment and in patients on dialysis.
Elderly Patients:
No dedicated clinical studies have been conducted to evaluate pharmacokinetics of lenalidomide in the elderly. Population pharmacokinetic analyses included patients with ages ranging from 39 to 85 years old and show that age does not influence the disposition of lenalidomide.
Patients with Hepatic Disease:
Population pharmacokinetic analyses included patients with mild hepatic impairment (N = 16, total bilirubin >1 to = 1.5 x ULN or AST > ULN) and show that mild hepatic impairment does not influence the disposition of lenalidomide. There are no data available for patients with moderate to severe hepatic impairment.
Pediatric:
No pharmacokinetic data are available in patients below the age of 18 years.
Other Intrinsic Factors:
Population pharmacokinetic analyses show that body weight (33-135 kg), gender, race, and type of hematological malignancies (MM, MDS or MCL) do not have a clinically relevant effect on lenalidomide clearance in adult patients.
NONCLINICAL TOXICOLOGY
Carcinogenesis, mutagenesis, impairment of fertility
Carcinogenicity studies with lenalidomide have not been conducted.
Lenalidomide was not mutagenic in the bacterial reverse mutation assay (Ames test) and did not induce chromosome aberrations in cultured human peripheral blood lymphocytes, or mutations at the thymidine kinase (tk) locus of mouse lymphoma L5178Y cells. Lenalidomide did not increase morphological transformation in Syrian Hamster Embryo assay or induce micronuclei in the polychromatic erythrocytes of the bone marrow of male rats.
A fertility and early embryonic development study in rats, with administration of lenalidomide up to 500 mg/kg (approximately 200 times the human dose of 25 mg, based on body surface area) produced no parental toxicity and no adverse effects on fertility.
CLINICAL STUDIES
Multiple Myeloma
Two randomized studies (Studies 1 and 2) were conducted to evaluate the efficacy and safety of Lenalidomide. These multicenter, multinational, double-blind, placebo-controlled studies compared Lenalidomide plus oral pulse high-dose dexamethasone therapy to dexamethasone therapy alone in patients with multiple myeloma who had received at least one prior treatment. These studies enrolled patients with absolute neutrophil counts (ANC) = 1000/mm3, platelet counts = 75,000/mm3, serum creatinine = 2.5 mg/dL, serum SGOT/AST or SGPT/ALT = 3 x upper limit of normal (ULN), and serum direct bilirubin = 2 mg/dL
In both studies, patients in the Lenalidomide/dexamethasone group took 25 mg of Lenalidomide orally once daily on Days 1 to 21 and a matching placebo capsule once daily on Days 22 to 28 of each 28-day cycle. Patients in the placebo/dexamethasone group took 1 placebo capsule on Days 1 to 28 of each 28-day cycle. Patients in both treatment groups took 40 mg of dexamethasone orally once daily on Days 1 to 4, 9 to 12, and 17 to 20 of each 28-day cycle for the first 4 cycles of therapy.
The dose of dexamethasone was reduced to 40 mg orally once daily on Days 1 to 4 of each 28-day cycle after the first 4 cycles of therapy. In both studies, treatment was to continue until disease progression.
In both studies, dose adjustments were allowed based on clinical and laboratory findings. Sequential dose reductions to 15 mg daily, 10 mg daily and 5 mg daily were allowed for toxicity.
Table 8 summarizes the baseline patient and disease characteristics in the two studies. In both studies, baseline demographic and disease-related characteristics were comparable between the Lenalidomide/dexamethasone and placebo/dexamethasone groups.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Lenalidomide/Dex N=177 |
Placebo/Dex N=176 |
Lenalidomide/Dex N=176 |
Placebo/Dex N=175 |
|
| Patient Characteristics | ||||
| Age (years) Median Min, Max |
64 36, 86 | 62 37, 85 | 63 33, 84 | 64 40, 82 |
| Sex Male Female | 106 (60%) 71 (40%) | 104 (59%) 72 (41%) | 104 (59%) 72 (41%) | 103 (59%) 72 (41%) |
| Race/Ethnicity White Other | 141(80%) 36 (20%) | 148 (84%) 28 (16%) | 172 (98%) 4 (2%) | 175(100%) 0 (0%) |
| ECOG Performance Status 0-1 | 157 (89%) | 168 (95%) | 150 (85%) | 144 (82%) |
| Disease Characteristics | ||||
| Multiple Myeloma Stage (Durie-Salmon) I II III | 3% 32% 64% | 3% 31% 66% | 6% 28% 65% | 5% 33% 63% |
| B2-microglobulin (mg/L) = 2.5 mg/L >2.5 mg/L | 52 (29%) 125 (71%) | 51 (29%) 125 (71%) | 51 (29%) 125 (71%) | 48 (27%) 127 (73%) |
| Number of Prior Therapies | ||||
| 1 = 2 | 38% 62% | 38% 62% | 32% 68% | 33% 67% |
| Types of Prior Therapies | ||||
| Stem Cell Transplantation | 62% | 61% | 55% | 54% |
| Thalidomide | 42% | 46% | 30% | 38% |
| Dexamethasone | 81% | 71% | 66% | 69% |
| Bortezomib | 11% | 11% | 5% | 4% |
| Melphalan | 33% | 31% | 56% | 52% |
| Doxorubicin | 55% | 51% | 56% | 57% |
The primary efficacy endpoint in both studies was time to progression (TTP). TTP was defined as the time from randomization to the first occurrence of progressive disease.
Preplanned interim analyses of both studies showed that the combination of Lenalidomide/dexamethasone was significantly superior to dexamethasone alone for TTP. The studies were unblinded to allow patients in the placebo/dexamethasone group to receive treatment with the Lenalidomide/dexamethasone combination. For both studies, the extended follow-up survival data with crossovers were analyzed. In study 1, the median survival time was 39.4 months (95%CI: 32.9, 47.4) in Lenalidomide/dexamethasone group and 31.6 months (95%CI: 24.1, 40.9) in placebo/dexamethasone group, with a hazard ratio of 0.79 (95% CI: 0.61-1.03). In study 2, the median survival time was 37.5 months (95%CI: 29.9, 46.6) in Lenalidomide/dexamethasone group and 30.8 months (95%CI: 23.5, 40.3) in placebo/dexamethasone group, with a hazard ratio of 0.86 (95% CI: 0.65-1.14).
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Lenalidomide/Dex N=177 | Placebo/Dex N=176 | Lenalidomide/Dex N=176 | Placebo/Dex N=175 | |
| TTP | ||||
| Events n (%) | 73 (41) | 120 (68) | 68 (39) | 130 (74) |
| Median TTP in months [95% CI] | 13.9 [9.5, 18.5] | 4.7 [3.7, 4.9] | 12.1 [9.5, NE] | 4.7 [3.8, 4.8] |
| Hazard Ratio [95% CI] | 0.285 [0.210, 0.386] | 0.324 [0.240, 0.438] |
||
| Log-rank Test p-value 3 | <0.001 | <0.001 | ||
| Response | ||||
| Complete Response (CR) n (%) | 23 (13) | 1 (1) | 27 (15) | 7 (4) |
| Partial Response (RR/PR) n (%) | 84 (48) | 33 (19) | 77 (44) | 34 (19) |
| Overall Response n (%) | 107 (61) | 34 (19) | 104 (59) | 41 (23) |
| p-value | <0.001 | <0.001 | ||
| Odds Ratio [95% CI] | 6.38 [3.95, 10.32] | 4.72 [2.98, 7.49] |
||
Figure 1: Kaplan-Meier Estimate of Time to Progression — Study 1

Figure 2: Kaplan-Meier Estimate of Time to Progression — Study 2

Myelodysplastic Syndromes (MDS) with a Deletion 5q Cytogenetic Abnormality
The efficacy and safety of Lenalidomide were evaluated in patients with transfusion-dependent anemia in low- or intermediate-1- risk MDS with a 5q (q31-33) cytogenetic abnormality in isolation or with additional cytogenetic abnormalities, at a dose of 10 mg once daily or 10 mg once daily for 21 days every 28 days in an open-label, single-arm, multi-center study. The major study was not designed nor powered to prospectively compare the efficacy of the 2 dosing regimens. Sequential dose reductions to 5 mg daily and 5 mg every other day, as well as dose delays, were allowed for toxicity.
This major study enrolled 148 patients who had RBC transfusion dependent anemia. RBC transfusion dependence was defined as having received = 2 units of RBCs within 8 weeks prior to study treatment. The study enrolled patients with absolute neutrophil counts (ANC) = 500/mm3, platelet counts = 50,000/mm3, serum creatinine = 2.5 mg/dL, serum SGOT/AST or SGPT/ALT = 3 x upper limit of normal (ULN), and serum direct bilirubin = 2 mg/dL. Granulocyte colony-stimulating factor was permitted for patients who developed neutropenia or fever in association with neutropenia. Baseline patient and disease-related characteristics are summarized in Table 10.
|
[a] IPSS Risk Category: Low (combined score = 0), Intermediate-1 (combined score = 0.5 to 1.0), Intermediate-2 (combined score = 1.5 to 2.0), High (combined score = 2.5); Combined score = (Marrow blast score + Karyotype score + Cytopenia score) [b] French-American-British (FAB) classification of MDS. |
|||
| Overall (N=148) |
|||
|---|---|---|---|
| Age (years) | |||
| Median | 71.0 | ||
| Min, Max | 37.0, 95.0 | ||
| Gender | n | (%) | |
| Male | 51 | (34.5) | |
| Female | 97 | (65.5) | |
| Race | n | (%) | |
| White | 143 | (96.6) | |
| Other | 5 | (3.4) | |
| Duration of MDS (years) | |||
| Median | 2.5 | ||
| Min, Max | 0.1, 20.7 | ||
| Del 5 (q31-33) Cytogenetic Abnormality | n | (%) | |
| Yes | 148 | (100.0) | |
| Other cytogenetic abnormalities | 37 | (25.2) | |
| IPSS Score [a] | n | (%) | |
| Low (0) | 55 | (37.2) | |
| Intermediate-1 (0.5-1.0) | 65 | (43.9) | |
| Intermediate-2 (1.5-2.0) | 6 | (4.1) | |
| High (=2.5) | 2 | (1.4) | |
| Missing | 20 | (13.5) | |
| FAB Classification [b] from central review | n | (%) | |
| RA | 77 | (52.0) | |
| RARS | 16 | (10.8) | |
| RAEB | 30 | (20.3) | |
| CMML | 3 | (2.0) | |
The frequency of RBC transfusion independence was assessed using criteria modified from the International Working Group (IWG) response criteria for MDS. RBC transfusion independence was defined as the absence of any RBC transfusion during any consecutive “rolling” 56 days (8 weeks) during the treatment period.
Transfusion independence was seen in 99/148 (67%) patients (95% CI [59, 74]). The median duration from the date when RBC transfusion independence was first declared (i.e., the last day of the 56-day RBC transfusion-free period) to the date when an additional transfusion was received after the 56-day transfusion-free period among the 99 responders was 44 weeks (range of 0 to >67 weeks). Ninety percent of patients who achieved a transfusion benefit did so by completion of three months in the study.
RBC transfusion independence rates were unaffected by age or gender.
The dose of Lenalidomide was reduced or interrupted at least once due to an adverse event in 118 (79.7%) of the 148 patients; the median time to the first dose reduction or interruption was 21 days (mean, 35.1 days; range, 2-253 days), and the median duration of the first dose interruption was 22 days (mean, 28.5 days; range, 2-265 days). A second dose reduction or interruption due to adverse events was required in 50 (33.8%) of the 148 patients. The median interval between the first and second dose reduction or interruption was 51 days (mean, 59.7 days; range, 15-205 days) and the median duration of the second dose interruption was 21 days (mean, 26 days; range, 2-148 days).
Mantle Cell Lymphoma
A multicenter, single-arm, open-label trial of single-agent lenalidomide was conducted to evaluate the safety and efficacy of lenalidomide in patients with mantle cell lymphoma who have relapsed after or were refractory to bortezomib or a bortezomib-containing regimen. Patients with a creatinine clearance >60 mL/min were given lenalidomide at a dose of 25 mg once daily for 21 days every 28 days. Patients with a creatinine clearance =30 mL/min and <60 mL/min were given lenalidomide at a dose of 10 mg once daily for 21 days every 28 days. Treatment was continued until disease progression, unacceptable toxicity, or withdrawal of consent.
The trial included patients who were at least 18 years of age with biopsy-proven MCL with measurable disease by CT scan. Patients were required to have received prior treatment with an anthracycline or mitoxantrone, cyclophosphamide, rituximab, and bortezomib, alone or in combination. Patients were required to have documented refractory disease (defined as without any response of PR or better during treatment with bortezomib or a bortezomib-containing regimen), or relapsed disease (defined as progression within one year after treatment with bortezomib or a bortezomib-containing regimen). At enrollment patients were to have an absolute neutrophil counts (ANC) =1500/ mm3, platelet counts = 60,000/mm3, serum SGOT/AST or SGPT/ALT =3x upper limit of normal (ULN) unless there was documented evidence of liver involvement by lymphoma, serum total bilirubin =1.5 x ULN except in cases of Gilbert’s syndrome or documented liver involvement by lymphoma, and calculated creatinine clearance (Cockcroft-Gault formula) =30 mL/min.
The median age was 67 years (43-83), 81% were male and 96% were Caucasian. The table below summarizes the baseline disease-related characteristics and prior anti-lymphoma therapy in the Mantle Cell Lymphoma trial.
|
a)ECOG = Eastern Cooperative Oncology Group b)MIPI = MCL International Prognostic Index c) High tumor burden is defined as at least one lesion that is =5 cm in diameter or 3 lesions that are =3 cm in diameter d) Bulky disease is defined as at least one lesion that is =7cm in the longest diameter |
|
| Baseline Disease Characteristics and Prior Anti - Lymphoma Treatment | Total Patients (N=134) |
|---|---|
| ECOG Performance Statusa n(%)
0 1 2 3 | 43 (32) 73 (54) 17 (13) 1 (<1) |
| Advanced MCL Stage, n (%) III IV | 27 (20) 97 (72) |
| High or Intermediate MIPI Score b, n(%) | 90 (67) |
| High Tumor Burdenc, n (%) | 77 (57) |
| Bulky Diseased, n(%) | 44 (33) |
| Extranodal Disease | 101 (75) |
| Number of Prior Systemic Anti-Lymphoma Therapies, n (%) Median (range) 1 2 3 = 4 | 4 (2, 10) 0 (0) 29 (22) 34 (25) 71 (53) |
| Number of Subjects Who Received Prior Regimen Containing, n (%): Anthracycline/mitoxantrone Cyclophosphamide Rituximab Bortezomib | 133 (99) 133 (99) 134 (100) 134 (100) |
| Refractory to Prior Bortezomib | 81 (60) |
| Refractory to Last Prior Therapy | 74 (55) |
| Prior Autologous Bone Marrow or Stem Cell Transplant, n (%) | 39 (29) |
The efficacy endpoints in the MCL trial were overall response rate (ORR) and duration of response (DOR). Response was determined based on review of radiographic scans by an independent review committee according to a modified version of the International Workshop Lymphoma Response Criteria (Cheson, 1999). The DOR is defined as the time from the initial response (at least PR) to documented disease progression. The efficacy results for the MCL population were based on all evaluable patients who received at least one dose of study drug and are presented in Table 12. The median time to response was 2.2 months (range 1.8 to 13 months).
| Response Analyses (N = 133) | N (%) | 95% CI |
|---|---|---|
| Overall Response Rate (IWRC) (CR + CRu +PR) Complete Response (CR + CRu) CR CRu Partial Response (PR) | 34 (26) 9 (7) 1 (1) 8 (6) 25 (19) | (18.4, 33.9) (3.1, 12.5) |
| Duration of Response (months) | Median | 95% CI |
| Duration of Overall Response (CR + CRu + PR) (N = 34) | 16.6 | (7.7, 26.7) |
REFERENCES
1. OSHA Hazardous Drugs. OSHA [Accessed on 29 January 2013, from http://www.osha.gov/SLTC/hazardousdrugs/index.html]
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
White and blue-green opaque hard capsules imprinted “TAJ” on one half and “2.5 mg” on the other half in black ink:
2.5 mg bottles of 28
2.5 mg bottles of 100
White opaque capsules imprinted “TAJ” on one half and “5 mg” on the other half in black ink:
5 mg bottles of 28
5 mg bottles of 100
Blue/green and pale yellow opaque capsules imprinted “TAJ” on one half and “10 mg” on the other half in black ink:
10 mg bottles of 28
10 mg bottles of 100
Powder blue and white opaque capsules imprinted “TAJ” on one half and “15 mg” on the other half in black ink:
15 mg bottles of 21
15 mg bottles of 100
Powder blue and blue-green opaque hard capsules imprinted “TAJ” on one half and “20 mg” on the other half in black ink.
20 mg bottles of 21
20 mg bottles of 100
White opaque capsules imprinted “TAJ” on one half and “25 mg” on the other half in black ink:
25 mg bottles of 21
25 mg bottles of 100
Storage
Store at 20°C - 25°C (68°F - 77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [See USP Controlled Room Temperature].
Handling and Disposal
Care should be exercised in the handling of Lenalidomide. Lenalidomide capsules should not be opened or crushed. If powder from Lenalidomide contacts the skin, wash the skin immediately and thoroughly with soap and water. If Lenalidomide contacts the mucous membranes, flush thoroughly with water.
Procedures for the proper handling and disposal of anticancer drugs should be considered. Several guidelines on the subject have been published.1
Dispense no more than a 28-day supply.
PATIENT COUNSELING INFORMATION
See FDA-approved Patient labeling (Medication Guide)
Embryo-Fetal Toxicity
Advise patients that Lenalidomide is contraindicated in pregnancy [see Contraindicatons]. Lenalidomide is a thalidomide analog and can cause serious birth defects or death to a developing baby. [see Warnings and Precautions and Use in Specific Populations].
- Advise females of reproductive potential that they must avoid pregnancy while taking Lenalidomide and for at least 4 weeks after completing therapy.
- Initiate Lenalidomide treatment in females of reproductive potential only following a negative pregnancy test.
- Advise females of reproductive potential of the importance of monthly pregnancy tests and the need to use two different forms of contraception including at least one highly effective form simultaneously during Lenalidomide therapy, during dose interruption and for 4 weeks after she has completely finished taking Lenalidomide. Highly effective forms of contraception other than tubal ligation include IUD and hormonal (birth control pills, injections, patch or implants) and a partner’s vasectomy. Additional effective contraceptive methods include latex or synthetic condom, diaphragm and cervical cap.
- Instruct patient to immediately stop taking Lenalidomide and contact her doctor if she becomes pregnant while taking this drug, if she misses her menstrual period, or experiences unusual menstrual bleeding, if she stops taking birth control, or if she thinks FOR ANY REASON that she may be pregnant.
- Advise patient that if her doctor is not available, she can call 0-000-000-0000 for information on emergency contraception [see Warnings and Precautions and Use in Specific Populations].
- Advise males to always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking Lenalidomide and for up to 28 days after discontinuing Lenalidomide, even if they have undergone a successful vasectomy.
- Advise male patients taking Lenalidomide that they must not donate sperm [see Warnings and Precautions and Use in Specific Populations].
- All patients must be instructed to not donate blood while taking Lenalidomide, during dose interruptions and for 1 month following discontinuation of Lenalidomide [see Warnings and Precautions and Use in Specific Populations].
Hematologic Toxicity
Inform patients that Lenalidomide is associated with significant neutropenia and thrombocytopenia [see Boxed Warnings and Warnings and Precautions].
Venous Thromboembolism
Inform patients that Lenalidomide/dexamethasone has demonstrated significant increased risk of DVT and PE in patients with multiple myeloma [see Boxed Warnings and Warning and Precautions].
Increased Mortality in Patients with CLL
Inform patients that Lenalidomide had increased mortality in patients with CLL and serious adverse cardiovascular reactions, including atrial fibrillation, myocardial infarction, and cardiac failure [see Warning and Precautions].
Second Primary Malignancies
Inform patients of the potential risk of developing second primary malignancies during treatment with Lenalidomide.
Hepatotoxicity
Inform patients of the risk of hepatotoxicity, including hepatic failure and death, and to report any signs and symptoms associated with this event to their healthcare provider for evaluation.
Allergic Reactions
Inform patients of the potential for allergic reactions including hypersensitivity, angioedema, Stevens Johnsons Syndrome, or toxic epidermal necrolysis if they had such a reaction to THALOMID and report symptoms associated with these events to their healthcare provider for evaluation.
Tumor Lysis Syndrome
Inform patients of the potential risk of tumor lysis syndrome and to report any signs and symptoms associated with this event to their healthcare provider for evaluation.
Tumor Flare Reaction
Inform patients of the potential risk of tumor flare reaction and to report any signs and symptoms associated with this event to their healthcare provider for evaluation.
Dosing Instructions
Inform patients to take Lenalidomide once daily at about the same time each day, either with or without food. The capsules should not be opened, broken, or chewed. Lenalidomide should be swallowed whole with water.
Instruct patients that if they miss a dose of Lenalidomide, they may still take it up to 12 hours after the time they would normally take it. If more than 12 hours have elapsed, they should be instructed to skip the dose for that day. The next day, they should take Lenalidomide at the usual time. Warn patients to not take 2 doses to make up for the one that they missed.
Product Glimpse
Description
We are committed to work towards a healthier and happier world. The company is an integrated, research based international pharmaceutical company, producing a wide range of quality, affordable generic (Lenalidomide 5mg/10mg Capsules) medicines, trusted by health-care professionals and patients across geographies.
We offer you the highest quality new Generic medicines ie. Lenalidomide 5mg/10mg Capsules, drugs and also with innovative packing at the lowest prices shipped to you from India. Browse our latest Pharmaceuticals and Generics possibilities and other pharmaceuticals possibilities…more.