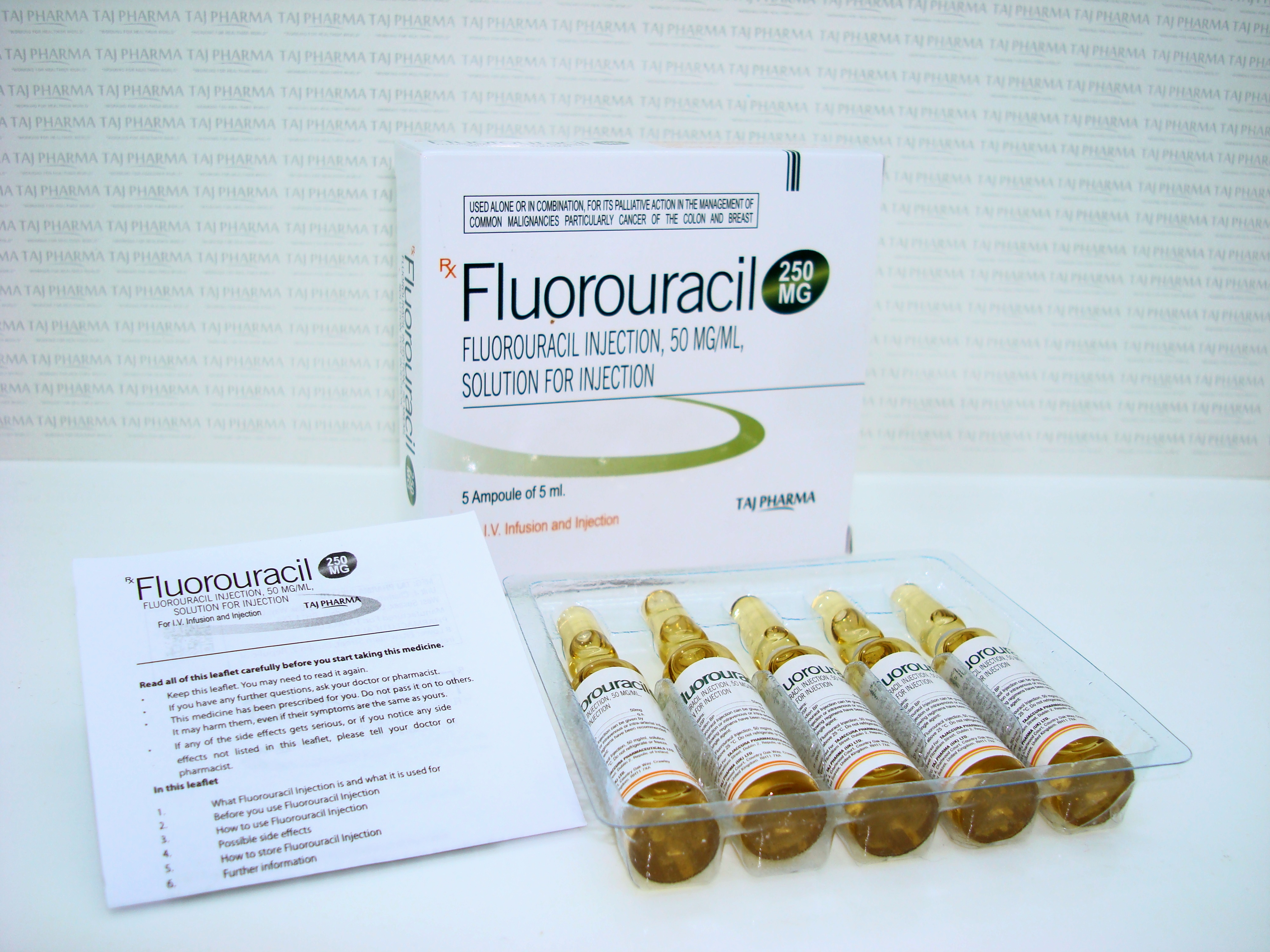
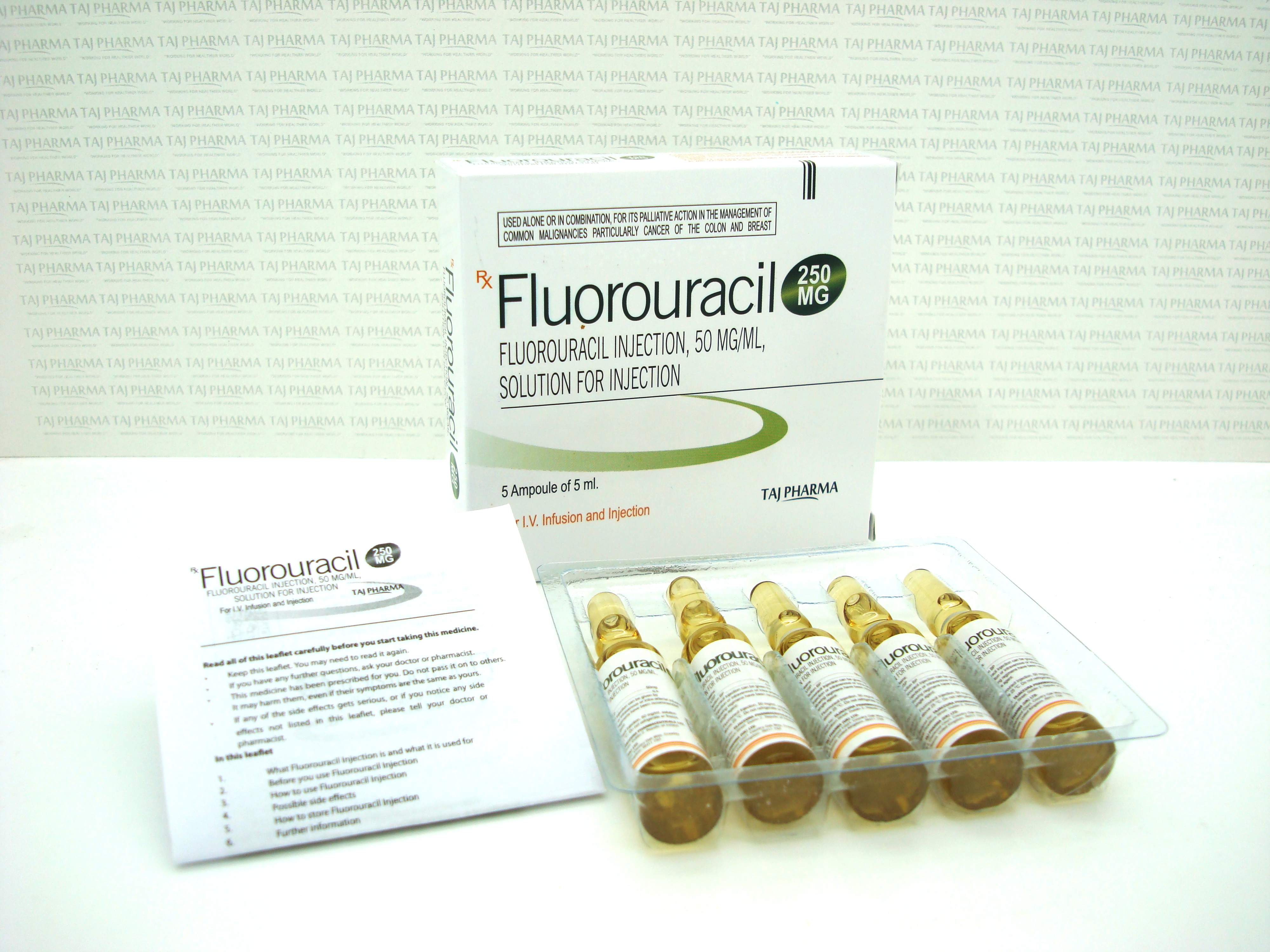
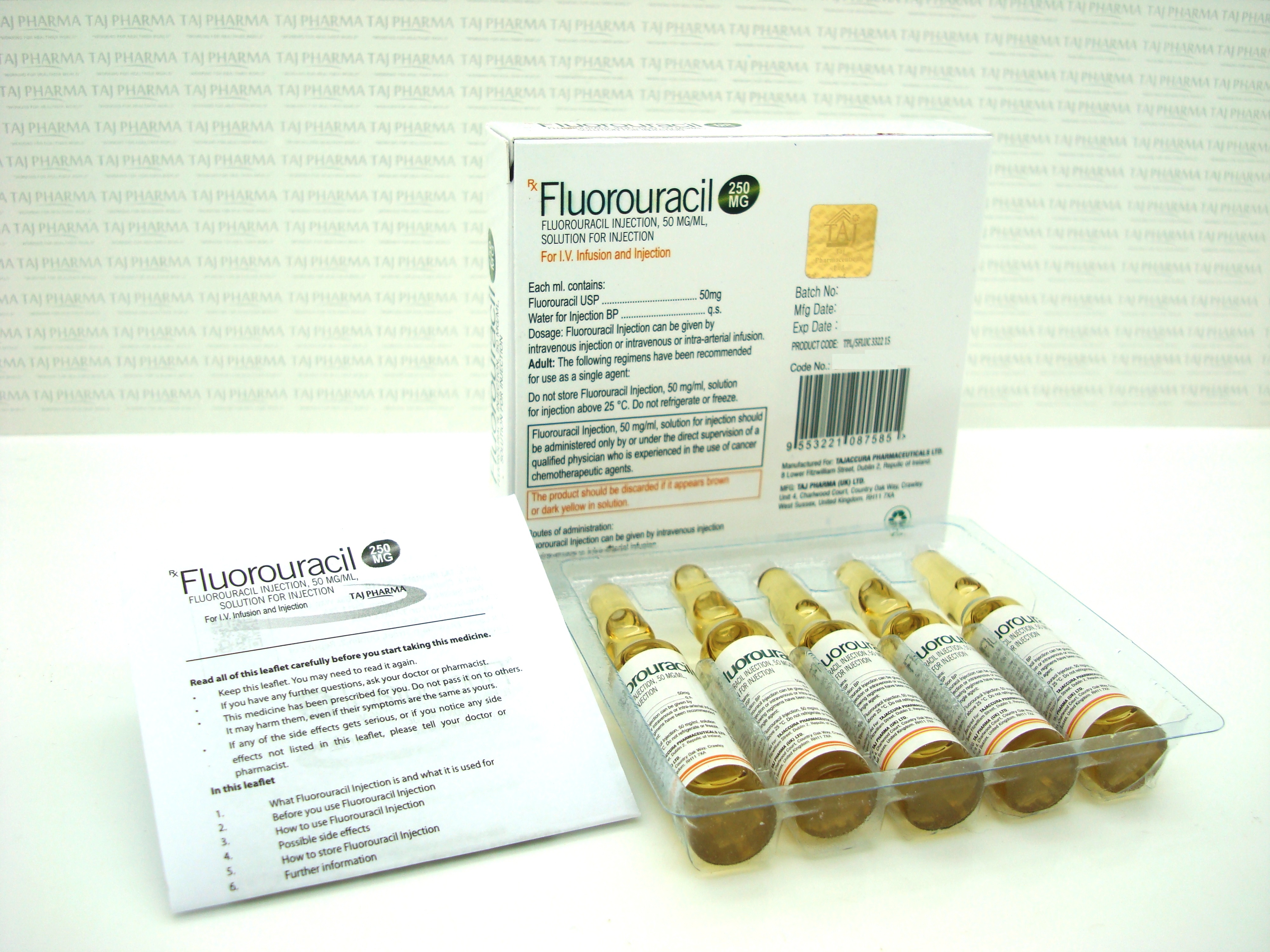
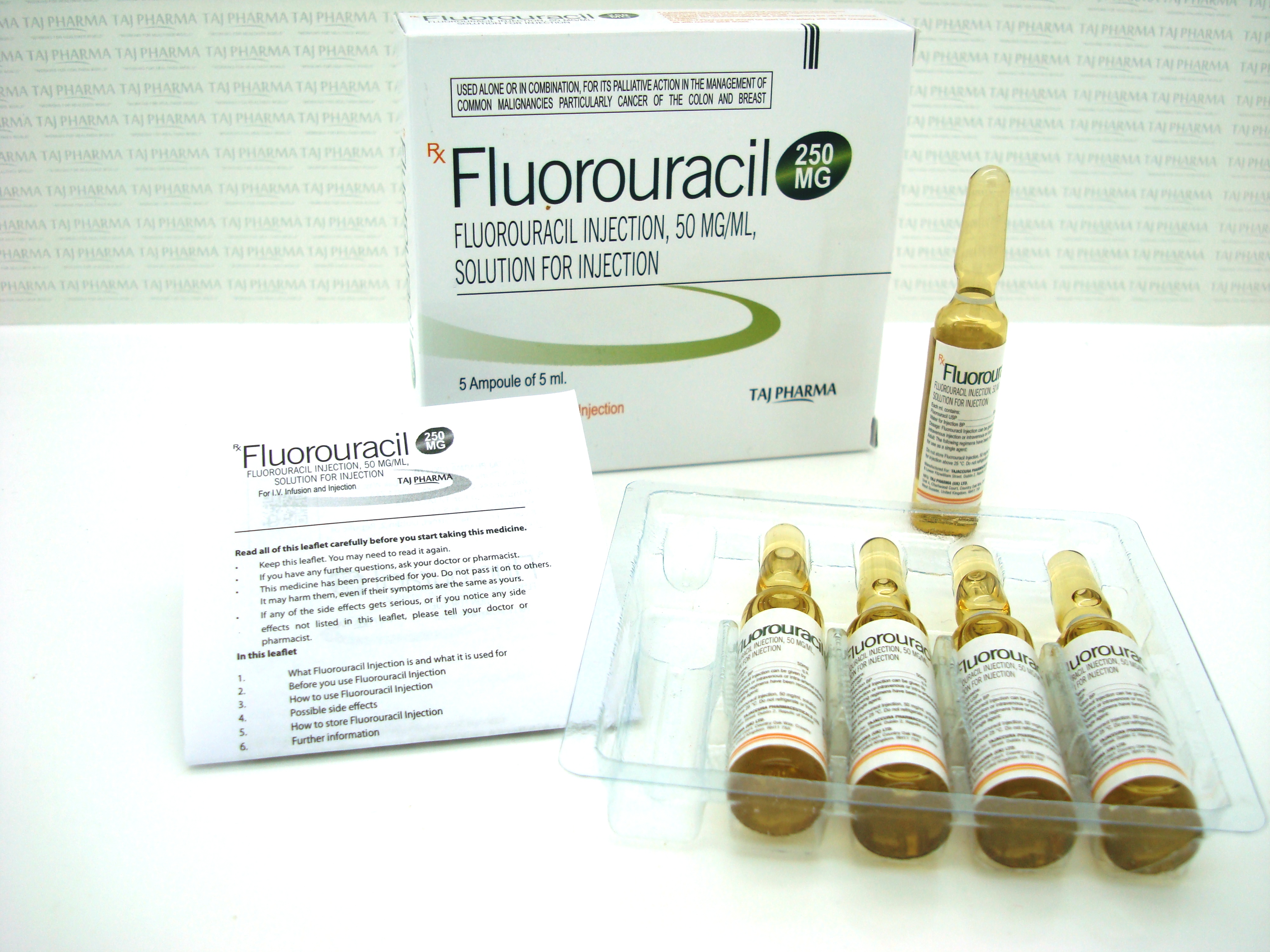
50 mg/mL (2.5 g/50 mL), Injection, USP
Fluorouracil-50mg/mL
50mg/mL (2.5 g/50 mL), Injection, USP
Sterile, Nonpyrogenic
Each mL contains: 50 mg fluorouracil;
pH is adjusted to approximately 9.2 with sodium hydroxide.
Usual Dosage: Swab stopper with an antiseptic solution. A single entry through the vial closure should be made utilizing a suitable sterile dispensing set or transfer device.
If a prompt fluid transfer is not possible, a maximum of 4 hours from the time of initial entry is allowed to complete the transferring operations. Discard the contents no later than 4 hours after initial closure puncture. (See Package Insert for complete dosage information and proper use of container.)
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. DO NOT FREEZE. PROTECT FROM LIGHT.
If a precipitate occurs due to exposure to low temperatures, resolubilize by heating to 140°F with vigorous shaking; allow to cool to body temperature before using.
Vial stoppers do not contain natural rubber latex.
What Fluorouracil Injection is and what it is used for
Fluorouracil Injection is an anti-cancer medicine. Treatment with an anti-cancer medicine is sometimes called cancer chemotherapy.
Fluorouracil Injection is used to treat many common cancers, particularly cancers of the large bowel and breast. It may be used in combination with other anti-cancer medicines or radiotherapy.
Before you use Fluorouracil Injection
Do not use Fluorouracil Injection
- if you have shown signs of hypersensitivity (severe allergy) to fluorouracil in the past
- if you are in a seriously weakened state due to long illness
- if your bone marrow has been damaged by other cancer treatments (including radiotherapy)
- if your cancer is non-malignant
Tell your doctor if any of the above applies to you before this medicine is used.
Take special care with Fluorouracil Injection
- if your bone marrow is not producing blood cells normally (your doctor will do a blood test to check this)
- if you have any problems with your kidneys
- if you have any problems with your liver including jaundice (yellowing of the skin)
- if you have suffered from angina (chest pain) or have a history of heart disease
- if you have reduced activity/deficiency of the enzyme DPD (dihydropyrimidine dehydrogenase)
- if you are in generally poor health and have lost a lot of weight
- if you have had surgery within the last 30 days
Tell your doctor if any of the above applies to you before this medicine is used.
Taking/using other medicines
Special care is needed if you are taking/using other medicines as some could interact with Fluorouracil Injection, for example:
- methotrexate (an anti-cancer medicine)
- metronidazole (an antibiotic)
- calcium leucovorin (also called calcium folinate - used to reduce the harmful effects of anti-cancer medicines)
- allopurinol (used to treat gout)
- cimetidine (used to treat stomach ulcers)
- warfarin (used to treat blood clots)
- sorivudine (an antiviral)
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
Tell your doctor if you are pregnant, trying to become pregnant or breast-feeding.
This medicine must not be used during pregnancy and breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Do not drive or use machines if you experience any side effect (e.g. visual disturbance) which may lessen your ability to do so.
Important information about one of the ingredients of Fluorouracil Injection
This medicinal product contains 7 mmol (160 mg) sodium per 1 gram dose. To be taken into consideration by patients on a controlled sodium diet.
How to use Fluorouracil Injection
This medicine is given into a vein or an artery. If it is given into a vein, it can either be injected (using a syringe) or infused (using a drip). If it is given into an artery, it will be given as an infusion.
If it is to be given as an infusion the medicine will be diluted before use.
Dose
Your doctor will work out the correct dose of Fluorouracil Injection for you and how often it must be given.
The dose of medicine given to you will depend on your medical condition, your size, if you have had recent surgery and how well your bone marrow, liver and kidneys are working.
Your doctor will tell how well your bone marrow, liver and kidneys are working using blood tests.
The total daily dose should not exceed 1 gram.
If you are given too much or too little Fluorouracil Injection
This medicine will be given to you by a doctor or nurse. It is unlikely that you will be given too much or too little, however, tell your doctor or nurse if you have any concerns.
Possible side effects
Like all medicines, fluorouracil can cause side effects, although not everybody gets them.
If any of the following happen, tell your doctor immediately:
- severe allergic reaction – you may experience a sudden itchy rash (hives), swelling of the hands, feet, ankles, face, lips, mouth or throat (which may cause difficulty in swallowing or breathing), and you may feel you are going to faint.
- chest pains
- your bowel motions are bloodstained or black
- your mouth becomes sore or develops ulcers
- symptoms of leucoencephalopathy (disease of brain) – weakness, coordination problems in arms and legs; thinking/speech difficulties; vision/memory problems; seizures; headaches.
These are very serious side effects. You may need urgent medical attention.
If you experience any of the following tell your doctor as soon as possible:
- quickening of your heart rate and breathlessness
- painful and/or watering eyes, changes in vision or sensitivity to light
- numbness, tingling or tremor in the hands or feet
- diarrhoea
- feeling confused
- feeling unsteady on your feet
- fever
- reddening of the palms of the hands and/or the soles of the feet
- skin problems
- changes in your nails
- the vein where fluorouracil is administered may become painful or discoloured
- hair loss (especially in women)
- feeling or being sick
Fluorouracil may lead to changes in your blood cells. Your doctor will take blood samples to check for these.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
How to store Fluorouracil Injection
Keep out of the reach and sight of children
Expiry
This medicine must not be used after the expiry date which is stated on the vial label and carton after 'EXP'. Where only a month and year is stated, the expiry date refers to the last day of that month.
Storage
Keep the vials in the outer carton, in order to protect from light and store at or below 25°C. They should not be refrigerated or frozen.
Prepared infusions should be used immediately, however, if this is not possible they can be stored for up to 5 days provided they have been prepared in a way to exclude microbial contamination.
Visible signs of deterioration
The product should be discarded if it appears brown or dark yellow in colour.
Disposal
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Further information
What Fluorouracil Injection contains
The active substance is fluorouracil. Each millilitre (ml) of solution contains 50 mg of fluorouracil.
The other ingredients are sodium hydroxide and Water for Injections.
What Fluorouracil Injection looks like and contents of the pack
Fluorouracil Injection is a clear, colourless or slightly yellow solution for injection which comes in glass containers called vials.
It may be supplied in packs containing:
- 5 x 250 mg/5 ml vials
- 5 x 500 mg/10 ml vials
- 5 x 1 g/20 ml vials
- 1 or 10 x 2.5 g/50 ml vial
- 1 x 5 g/100 ml vial
- 1 x 500 mg/10 ml ONCO-VIAL®
- 1 x 1 g/20 ml ONCO-VIAL®
- 1 x 2.5 g/50 ml ONCO-VIAL®
Not all packs may be marketed.
Manufacturer
Taj Pharma India (oncology)
India
DESCRIPTION
Fluorouracil Injection, USP, an antineoplastic antimetabolite, is a colorless to yellow aqueous sterile, nonpyrogenic injectable solution for intravenous administration. Each mL contains: 50 mg fluorouracil; pH is adjusted to approximately 9.2 with sodium hydroxide.
Chemically, fluorouracil, a fluorinated pyrimidine, is 5-fluoro-2,4 (1H,3H)-pyrimidinedione. It is a white to practically white crystalline powder which is sparingly soluble in water. The molecular weight of fluorouracil is 130.08 and the structural formula is:
A pharmacy bulk package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion or the filling of empty sterile syringes for patients with individualized dosing requirements.
CLINICAL PHARMACOLOGY
There is evidence that the metabolism of fluorouracil in the anabolic pathway blocks the methylation reaction of deoxyuridylic acid to thymidylic acid. In this manner, fluorouracil interferes with the synthesis of deoxyribonucleic acid (DNA) and to a lesser extent inhibits the formation of ribonucleic acid (RNA). Since DNA and RNA are essential to cell division and growth, the effect of fluorouracil may be to create a thymine deficiency which provokes unbalanced growth and death of the cell. The effects of DNA and RNA deprivation are most marked on those cells which grow more rapidly and which take up fluorouracil at a more rapid rate.
Following intravenous injection, fluorouracil distributes into tumors, intestinal mucosa, bone marrow, liver and other tissues throughout the body. In spite of its limited lipid solubility, fluorouracil diffuses readily across the blood-brain barrier and distributes into cerebrospinal fluid and brain tissue.
Seven to 20 percent of the parent drug is excreted unchanged in the urine in 6 hours; of this, over 90% is excreted in the first hour. The remaining percentage of the administered dose is metabolized, primarily in the liver. The catabolic metabolism of fluorouracil results in degradation products (e.g., CO2, urea and α-fluoro-ß-alanine) which are inactive. The inactive metabolites are excreted in the urine over the next 3 to 4 hours. When fluorouracil is labeled in the six carbon position, thus preventing the 14C metabolism to CO2, approximately 90% of the total radioactivity is excreted in the urine. When fluorouracil is labeled in the two carbon position, approximately 90% of the total radioactivity is excreted in expired CO2. Ninety percent of the dose is accounted for during the first 24 hours following intravenous administration.
Following intravenous administration of fluorouracil, the mean half-life of elimination from plasma is approximately 16 minutes, with a range of 8 to 20 minutes, and is dose dependent. No intact drug can be detected in the plasma 3 hours after an intravenous injection.
INDICATIONS AND USAGE
Fluorouracil is effective in the palliative management of carcinoma of the colon, rectum, breast, stomach and pancreas.
CONTRAINDICATIONS
Fluorouracil therapy is contraindicated for patients in a poor nutritional state, those with depressed bone marrow function, those with potentially serious infections or those with a known hypersensitivity to Fluorouracil.
WARNINGS
THE DAILY DOSE OF FLUOROURACIL IS NOT TO EXCEED 800 MG. IT IS RECOMMENDED THAT PATIENTS BE HOSPITALIZED DURING THEIR FIRST COURSE OF TREATMENT.
Fluorouracil should be used with extreme caution in poor risk patients with a history of high-dose pelvic irradiation or previous use of alkylating agents, those who have widespread involvement of bone marrow by metastatic tumors or those with impaired hepatic or renal function.
Rarely, unexpected, severe toxicity (e.g., stomatitis, diarrhea, neutropenia and neurotoxicity) associated with 5-fluorouracil has been attributed to deficiency of dipyrimidine dehydrogenase activity.1 A few patients have been rechallenged with 5-fluorouracil and despite 5-fluorouracil dose lowering, toxicity recurred and progressed with worse morbidity. Absence of this catabolic enzyme appears to result in prolonged clearance of 5-fluorouracil.
PregnancyTeratogenic Effects: Pregnancy Category D.
Fluorouracil may cause fetal harm when administered to a pregnant woman. Fluorouracil has been shown to be teratogenic in laboratory animals. Fluorouracil exhibited maximum teratogenicity when given to mice as single intraperitoneal injections of 10 to 40 mg/kg on day 10 or 12 of gestation. Similarly, intraperitoneal doses of 12 to 37 mg/kg given to rats between days 9 and 12 of gestation and intramuscular doses of 3 to 9 mg given to hamsters between days 8 and 11 of gestation were teratogenic. Malformations included cleft palates, skeletal defects and deformed appendages, paws and tails. The dosages which were teratogenic in animals are 1 to 3 times the maximum recommended human therapeutic dose. In monkeys, divided doses of 40 mg/kg given between days 20 and 24 of gestation were not teratogenic.
There are no adequate and well-controlled studies with Fluorouracil in pregnant women. While there is no evidence of teratogenicity in humans due to Fluorouracil, it should be kept in mind that other drugs which inhibit DNA synthesis (e.g., methotrexate and aminopterin) have been reported to be teratogenic in humans. Women of childbearing potential should be advised to avoid becoming pregnant. If the drug is used during pregnancy, or if the patient becomes pregnant while taking the drug, the patient should be told of the potential hazard to the fetus. Fluorouracil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Combination TherapyAny form of therapy which adds to the stress of the patient, interferes with nutrition or depresses bone marrow function will increase the toxicity of Fluorouracil.
PRECAUTIONS
GeneralFluorouracil is a highly toxic drug with a narrow margin of safety. Therefore, patients should be carefully supervised, since therapeutic response is unlikely to occur without some evidence of toxicity. Severe hematological toxicity, gastrointestinal hemorrhage and even death may result from the use of Fluorouracil despite meticulous selection of patients and careful adjustment of dosage. Although severe toxicity is more likely in poor risk patients, fatalities may be encountered occasionally even in patients in relatively good condition.
Therapy is to be discontinued promptly whenever one of the following signs of toxicity appears:
Stomatitis or esophagopharyngitis, at the first visible sign.
Leukopenia (WBC under 3500) or a rapidly falling white blood count.
Vomiting, intractable.
Diarrhea, frequent bowel movements or watery stools.
Gastrointestinal ulceration and bleeding.
Thrombocytopenia (platelets under 100,000).
Hemorrhage from any site.
The administration of 5-fluorouracil has been associated with the occurrence of palmar-plantar erythrodysesthesia syndrome, also known as hand-foot syndrome. This syndrome has been characterized as a tingling sensation of hands and feet which may progress over the next few days to pain when holding objects or walking. The palms and soles become symmetrically swollen and erythematous with tenderness of the distal phalanges, possibly accompanied by desquamation. Interruption of therapy is followed by gradual resolution over 5 to 7 days. Although pyridoxine has been reported to ameliorate the palmarplantar erythrodysesthesia syndrome, its safety and effectiveness have not been established.
Information for PatientsPatients should be informed of expected toxic effects, particularly oral manifestations. Patients should be alerted to the possibility of alopecia as a result of therapy and should be informed that it is usually a transient effect.
Laboratory TestsWhite blood counts with differential are recommended before each dose.
Drug InteractionsLeucovorin calcium may enhance the toxicity of fluorouracil.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of fluorouracil have not been conducted. However, there was no evidence of carcinogenicity in small groups of rats given fluorouracil orally at doses of 0.01, 0.3, 1 or 3 mg per rat 5 days per week for 52 weeks, followed by a 6-month observation period. Also, in other studies, 33 mg/kg of fluorouracil was administered intravenously to male rats once a week for 52 weeks followed by observation for the remainder of their lifetimes with no evidence of carcinogenicity. Female mice were given 1 mg of fluorouracil intravenously once a week for 16 weeks with no effect on the incidence of lung adenomas. On the basis of the available data, no evaluation can be made of the carcinogenic risk of fluorouracil to humans.
Mutagenesis
Oncogenic transformation of fibroblasts from mouse embryo has been induced in vitro by fluorouracil, but the relationship between oncogenicity and mutagenicity is not clear. Fluorouracil has been shown to be mutagenic to several strains of Salmonella typhimurium, including TA 1535, TA 1537 and TA 1538, and to Saccharomyces cerevisiae, although no evidence of mutagenicity was found with Salmonella typhimurium strains TA 92, TA 98 and TA 100. In addition, a positive effect was observed in the micronucleus test on bone marrow cells of the mouse, and fluorouracil at very high concentrations produced chromosomal breaks in hamster fibroblasts in vitro.
Impairment of Fertility
Fluorouracil has not been adequately studied in animals to permit an evaluation of its effects on fertility and general reproductive performance. However, doses of 125 or 250 mg/kg, administered intraperitoneally, have been shown to induce chromosomal aberrations and changes in chromosomal organization of spermatogonia in rats. Spermatogonial differentiation was also inhibited by fluorouracil, resulting in transient infertility. However, in studies with a strain of mouse which is sensitive to the induction of sperm head abnormalities after exposure to a range of chemical mutagens and carcinogens, fluorouracil did not produce any abnormalities at oral doses of up to 80 mg/kg/day. In female rats, fluorouracil, administered intraperitoneally at weekly doses of 25 or 50 mg/kg for 3 weeks during the pre-ovulatory phase of oogenesis, significantly reduced the incidence of fertile matings, delayed the development of pre- and post-implantation embryos, increased the incidence of pre-implantation lethality and induced chromosomal anomalies in these embryos. In a limited study in rabbits, a single 25 mg/kg dose of fluorouracil or 5 daily doses of 5 mg/kg had no effect on ovulation, appeared not to affect implantation and had only a limited effect in producing zygote destruction. Compounds such as fluorouracil, which interfere with DNA, RNA and protein synthesis, might be expected to have adverse effects on gametogenesis.
Pregnancy Category D.
Nonteratogenic Effects
Fluorouracil has not been studied in animals for its effects on peri- and postnatal development. However, fluorouracil has been shown to cross the placenta and enter into fetal circulation in the rat. Administration of fluorouracil has resulted in increased resorptions and embryolethality in rats. In monkeys, maternal doses higher than 40 mg/kg resulted in abortion of all embryos exposed to fluorouracil. Compounds which inhibit DNA, RNA and protein synthesis might be expected to have adverse effects on peri- and postnatal development.
It is not known whether fluorouracil is excreted in human milk. Because fluorouracil inhibits DNA, RNA and protein synthesis, mothers should not nurse while receiving this drug.
Pediatric UseSafety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
Stomatitis and esophagopharyngitis (which may lead to sloughing and ulceration), diarrhea, anorexia, nausea and emesis are commonly seen during therapy.
Leukopenia usually follows every course of adequate therapy with Fluorouracil. The lowest white blood cell counts are commonly observed between the 9th and 14th days after the first course of treatment, although uncommonly the maximal depression may be delayed for as long as 20 days. By the 30th day the count has usually returned to the normal range.
Alopecia and dermatitis may be seen in a substantial number of cases. The dermatitis most often seen is a pruritic maculopapular rash usually appearing on the extremities and less frequently on the trunk. It is generally reversible and usually responsive to symptomatic treatment.
Other adverse reactions are:
Hematologic: pancytopenia, thrombocytopenia, agranulocytosis, anemia.
Cardiovascular: myocardial ischemia, angina.
Gastrointestinal: gastrointestinal ulceration and bleeding.
Allergic Reactions: anaphylaxis and generalized allergic reactions.
Neurologic: acute cerebellar syndrome (which may persist following discontinuance of treatment), nystagmus, headache.
Dermatologic: dry skin; fissuring; photosensitivity, as manifested by erythema or increased pigmentation of the skin; vein pigmentation, palmar-plantar erythrodysesthesia syndrome, as manifested by tingling of the hands and feet followed by pain, erythema and swelling.
Ophthalmic: lacrimal duct stenosis, visual changes, lacrimation, photophobia.
Psychiatric: disorientation, confusion, euphoria.
Miscellaneous: thrombophlebitis, epistaxis, nail changes (including loss of nails).
OVERDOSAGE
The possibility of overdosage with Fluorouracil is unlikely in view of the mode of administration. Nevertheless, the anticipated manifestations would be nausea, vomiting, diarrhea, gastrointestinal ulceration and bleeding, bone marrow depression (including thrombocytopenia, leukopenia and agranulocytosis). No specific antidotal therapy exists. Patients who have been exposed to an overdose of Fluorouracil should be monitored hematologically for at least four weeks. Should abnormalities appear, appropriate therapy should be utilized.
The acute intravenous toxicity of fluorouracil is as follows:
| Species | LD50(mg/kg ± S.E.) |
|---|---|
|
Mouse Rat Rabbit Dog |
340 ± 17 165 ± 26 27 ± 5.1 31.5 ± 3.8 |
DOSAGE AND ADMINISTRATION
General InstructionsFluorouracil Injection, USP should be administered only intravenously, using care to avoid extravasation. No dilution is required.
All dosages are based on the patient’s actual weight. However, the estimated lean body mass (dry weight) is used if the patient is obese or if there has been a spurious weight gain due to edema, ascites or other forms of abnormal fluid retention.
It is recommended that prior to treatment each patient be carefully evaluated in order to estimate as accurately as possible the optimum initial dosage of Fluorouracil.
Dosage12 mg/kg are given intravenously once daily for 4 successive days. The daily dose should not exceed 800 mg. If no toxicity is observed, 6 mg/kg are given on the 6th, 8th, 10th and 12th days unless toxicity occurs. No therapy is given on the 5th, 7th, 9th or 11th days. Therapy is to be discontinued at the end of the 12th day, even if no toxicity has become apparent.
Poor risk patients or those who are not in an adequate nutritional state should receive 6 mg/kg/day for 3 days. If no toxicity is observed, 3 mg/kg may be given on the 5th, 7th and 9th days unless toxicity occurs. No therapy is given on the 4th, 6th or 8th days. The daily dose should not exceed 400 mg.
A sequence of injections on either schedule constitutes a “course of therapy.”
Maintenance TherapyIn instances where toxicity has not been a problem, it is recommended that therapy be continued using either of the following schedules:
- Repeat dosage of first course every 30 days after the last day of the previous course of treatment.
- When toxic signs resulting from the initial course of therapy have subsided, administer a maintenance dosage of 10 to 15 mg/kg/week as a single dose. Do not exceed 1 g per week.
The patient’s reaction to the previous course of therapy should be taken into account in determining the amount of the drug to be used, and the dosage should be adjusted accordingly. Some patients have received from 9 to 45 courses of treatment during periods which ranged from 12 to 60 months.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.2-7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
NoteParenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Although the Fluorouracil solution may discolor slightly during storage, the potency and safety are not adversely affected. If a precipitate occurs due to exposure to low temperatures, resolubilize by heating to 140°F and shaking vigorously; allow to cool to body temperature before using.
DIRECTION FOR PROPER USE OF PHARMACY BULK PACKAGE
Pharmacy Bulk Packages are for use in a Pharmacy Admixture Service only in a vertical laminar flow hood. They should be inserted into the ring sling (plastic hanging device) provided and suspended as a unit in the vertical laminar flow hood. The container closure should be penetrated only one time utilizing a suitable sterile dispensing set or transfer device which allows measured distribution of the contents. Swab vial stopper with an antiseptic solution. Insert the device/set into the vial using aseptic technique (see graphic illustration below).
Once the sterile dispensing set or transfer device has been inserted into the container, withdrawal of the contents should be accomplished without delay. However, if this is not possible, a maximum time of 4 hours from the initial entry may be allowed to complete fluid aliquoting/transferring operations. The transferred drug solution should be used promptly. Discard the contents no later than 4 hours after initial closure puncture.
HOW SUPPLIED
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
DO NOT FREEZE. PROTECT FROM LIGHT.
Vial stoppers do not contain natural rubber latex.
Product Glimpse
Description
Fluorouracil Injection, USP, an antineoplastic antimetabolite, is a colorless to yellow aqueous sterile, nonpyrogenic injectable solution for intravenous administration. Each mL contains: 50 mg fluorouracil; pH is adjusted to approximately 9.2 with sodium hydroxide.
Chemically, fluorouracil, a fluorinated pyrimidine, is 5-fluoro-2,4 (1H,3H)-pyrimidinedione. It is a white to practically white crystalline powder which is sparingly soluble in water. The molecular weight of fluorouracil is 130.08 and the structural formula is:
A pharmacy bulk package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion or the filling of empty sterile syringes for patients with individualized dosing requirements.