Gefitinib 250mg Tablets
Gefitinib
Gefitinib 250mg Tablets
Gefitinib tablets are supplied as round, biconvex, brown film-coated tablets intagliated with "Gefitinib 250" on one side and plain on the other side,each containing 250 mg of gefitinib.
Bottles of 30 Tablets
Storage
Store at controlled room temperature 20-25°C (68-77°F) [see USP].
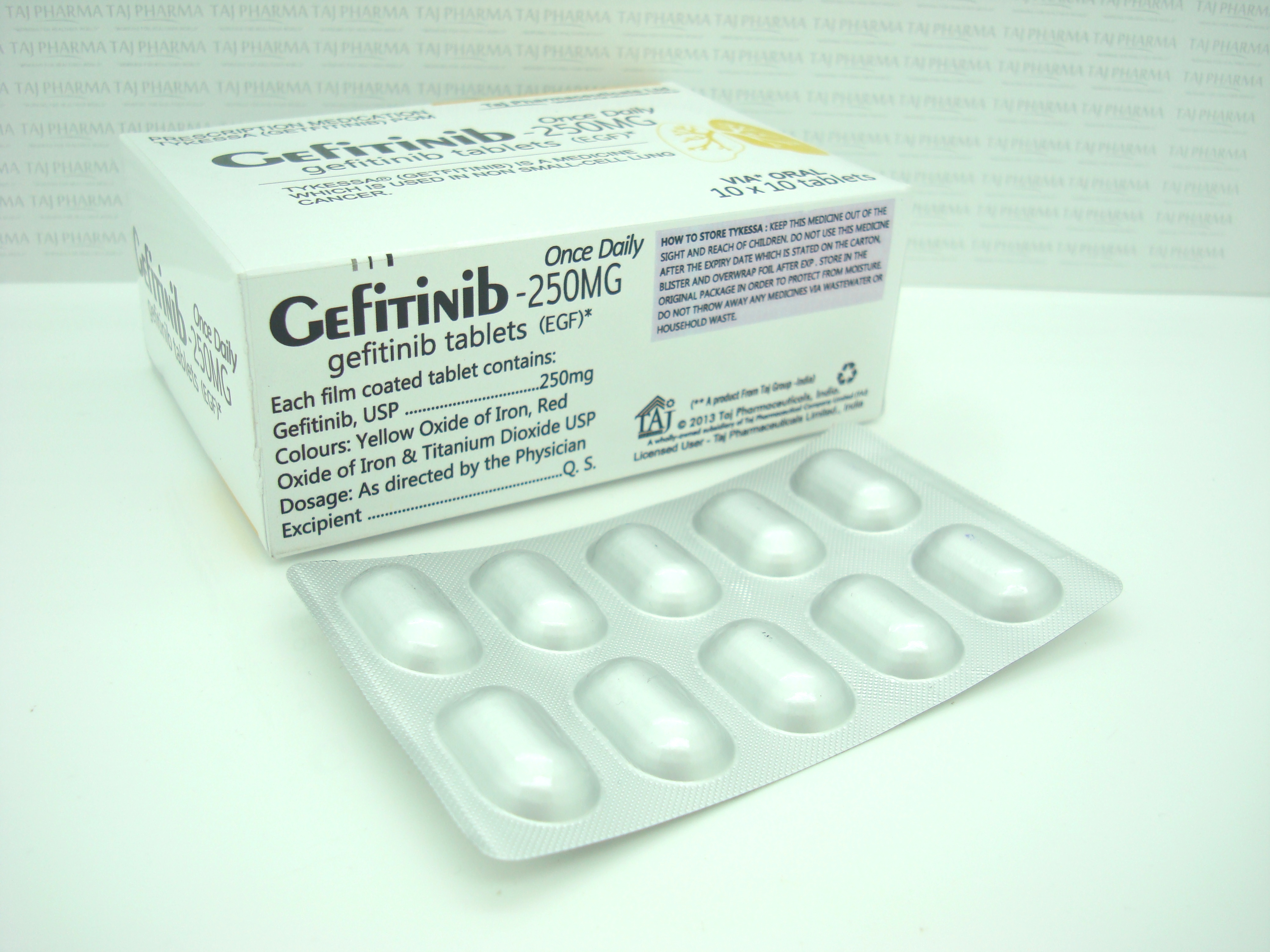
The inactive ingredients of Gefitinib tablets are: Tablet core: Lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, povidone, sodium lauryl sulfate and magnesium stearate. Coating: hypromellose, polyethylene glycol 300, titanium dioxide, red ferric oxide and yellow ferric oxide.
Gefitinib
Gefitinib 250mg Tablets
What is gefitinib?
Gefitinib is a type of treatment called a tyrosine kinase inhibitor. Kinases are important proteins in the body that regulate how the cells grow and divide.
How gefitinib works
Gefitinib works by blocking (inhibiting) signals within the cancer cells that make them grow and divide. Blocking the signals causes the cells to die.
When it is used
Gefitinib is used to treat some people with non-small cell lung cancer (NSCLC).
It only works for NSCLCs that have changes called EGFR mutations on the surface of their cells. EGFR mutations are most likely to occur in NSCLC in:
- women
- people who have never smoked
- people with adenocarcinoma (a specific type of NSCLC)
- people of Asian origin.
Tests may be done to check the level of EGFR. These will help you and your doctors decide if you're likely to benefit from gefitinib. Testing can be done at the time the cancer is diagnosed, or samples of cancer cells from previous biopsies or surgery may be used.
The National Institute for Health and Clinical Excellence (NICE) gives advice on which new drugs or treatments should be available on the NHS in England and Wales. It recommends gefitinib as a possible first treatment for people with NSCLC that is EGFR positive and has spread to surrounding tissues or other parts of the body advanced or metastatic).
Recommendations on the use of new drugs within the NHS in Scotland are made by the Scottish Medicines Consortium (SMC). The SMC doesn’t currently recommend the use of gefitinib. As a result, it may not be available on the NHS in Scotland, although you may be given it as part of a clinical trial.
If you live in Northern Ireland, speak to your cancer specialist about whether gefitinib is recommended to treat your type of cancer.
What gefitinib looks like
Gefitinib is a brown, round tablet, coated with a film.
How gefitinib is given
Gefitinib is taken as a tablet once a day. You should take the tablet at about the same time each day. It should be swallowed whole with a glass of water, and can be taken with or without food. If you have trouble swallowing tablets, the gefitinib can be put in a glass of water and left to dissolve. The tablet may take 20 minutes to completely dissolve and should be drunk straight away. The glass should be rinsed with more water and that should also be drunk.
Gefitinib is usually taken for as long as a person benefits from it.
Possible side effects of gefitinib
Each person’s reaction to cancer treatment is different. Some people have very few side effects while others may experience more. The side effects described here won't affect everyone having this treatment.
We've outlined the most common side effects but haven't included those that are rare and therefore unlikely to affect you. If you notice any effects that aren't listed here, discuss them with your doctor or specialist nurse.
Skin changes
The most common side effect of gefitinib is an acne-like rash that mainly affects the head, chest and back. This usually begins during the first 2-3 weeks of treatment and goes away once treatment ends.
Your skin may also become dry and itchy, or feel tender and peel. Some people find that the nails on their hands or feet become red, sore and brittle.
Taking the following steps may help reduce the severity of skin changes, although they can’t prevent them altogether:
- Use tepid water and mild, non-scented soap for bathing and washing.
- Avoid skincare products containing alcohol.
- Don’t use anti-acne products as they can dry your skin and make your symptoms worse.
- Moisturise your skin regularly and after bathing. Your doctor or specialist nurse can advise you on which moisturisers are best.
- Protect your hands and fingernails from detergents by wearing rubber gloves when washing dishes.
- Protect your skin in the sun, as sunlight can make skin symptoms worse. During treatment with gefitinib, and for several months afterwards, you will be more sensitive to the sun, and your skin may burn more easily than normal. You can still go out in the sun, but you should wear a suncream with a high sun protection factor (SPF), and cover up with clothing and a hat. If you’re having radiotherapy, don't apply suncream to any skin in the area being treated.
Let your doctor know as soon as possible if you develop skin or nail changes. They can prescribe treatment to help. In people who have more severe side effects, treatment with gefitinib can be stopped for a few days to allow their skin to recover.
Diarrhoea
Gefitinib can cause diarrhoea. This can usually be easily controlled with medicine, but tell your doctor if it's severe or continues. It's important to drink plenty of fluids if you have diarrhoea.
Loss of appetite
Some people lose their appetite while they’re having gefitinib. A dietitian or specialist nurse at your hospital can give advice about how to boost your appetite and maintain your weight.
Feeling sick (nausea) and being sick (vomiting)
Your doctor can prescribe very effective anti-sickness (anti-emetic) drugs to prevent or greatly reduce nausea or vomiting. If the sickness isn't controlled, or if it continues, tell your doctor as they can prescribe other anti-sickness drugs that may be more effective.
Some anti-sickness drugs can cause constipation. Let your doctor or nurse know if this is a problem.
Tiredness (fatigue)
Feeling tired is a common side effect of cancer treatment, especially towards the end of treatment and for some weeks after it’s over. It’s important to try to pace yourself and get as much rest as you need. Try to balance this with taking some gentle exercise, such as short walks, which will help. If tiredness is making you feel sleepy, don’t drive or operate machinery.
Liver changes
Treatment with gefitinib may cause changes in the way that your liver works, although it will return to normal when the treatment finishes. You're very unlikely to notice any problems but your doctor will take regular blood samples to check your liver is working properly.
Hair changes
Your eyelashes may grow longer and more curly than usual. Men may notice they have less beard growth. You may notice your head and body hair is finer, curlier or more brittle. Some people have hair thinning or hair loss. If this happens, it usually develops gradually. These changes are usually temporary and gradually improve once treatment is over.
Eye problems
If you notice any change to your vision or pain or redness of your eyes, let your doctor know immediately.
Bleeding problems
Let your doctor know if you have nosebleeds or notice blood in your urine while taking gefitinib.
Breathing problems
This is an uncommon side effect that may affect about 1 in 100 people taking gefitinib. If you become breathless, if your breathing worsens or if you have a cough or fever, tell your doctor straight away. It may mean you have an inflammation of the lungs called 'interstitial lung disease', which is potentially very serious. If you're worried about this potential side effect, talk to your doctor or nurse.
It’s important to let your doctor know straight away if you feel unwell or have any severe side effects, even if they’re not mentioned above.
Additional information
Other medicines
Some medicines, including those you can buy in a shop or chemist, can be harmful to take while you are having gefitinib. Tell your doctor about any medicines you are taking, including over-the-counter drugs, complementary therapies and herbal drugs.
Fertility
Your ability to become pregnant or father a child may be affected by having this treatment. It's important to discuss fertility with your doctor before starting treatment
Contraception
Little is known about the effects of gefitinib on a developing baby. Therefore, it’s not advisable to become pregnant or father a child while taking this drug.
It’s not known whether gefitinib is present in semen or vaginal fluids. To protect your partner, it’s safest to either avoid sex or use a barrier form of contraception for about 48 hours after treatment.
Breastfeeding
There is a potential risk that gefitinib may be present in breast milk, so women are advised not to breastfeed during treatment and for a few months afterwards.
Non-cancer hospital admission
If you’re admitted to hospital for a reason not related to the cancer, it’s important to tell the doctors and nurses looking after you that you‘re having gefitinib treatment. You should tell them the name of your cancer specialist so that they can ask them for advice.
Emergency contacts
It’s a good idea to know who you should contact if you have any problems or troublesome side effects when you’re at home. During office hours you can contact the clinic or ward where you had your treatment. Your specialist nurse or doctor will tell you who to contact during the evening or at weekends.
Gefitinib
Gefitinib 250mg Tablets
DESCRIPTION
Gefitinib Tablets contain 250 mg of gefitinib and are available as brown film-coated tablets for daily oral administration.
Gefitinib is an anilinoquinazoline with the chemical name 4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-4-morpholin) propoxy] and the following structural formula:

It has the molecular formula C22H24ClFN4O3, a relative molecular mass of 446.9 and is a white-colored powder. Gefitinib is a free base. The molecule has pKas of 5.4 and 7.2 and therefore ionizes progressively in solution as the pH falls. Gefitinib can be defined as sparingly soluble at pH 1, but is practically insoluble above pH 7, with the solubility dropping sharply between pH 4 and pH 6. In non-aqueous solvents, gefitinib is freely soluble in glacial acetic acid and dimethylsulphoxide, soluble in pyridine, sparingly soluble in tetrahydrofuran, and slightly soluble in methanol, ethanol (99.5%), ethyl acetate, propan-2-ol and acetonitrile.
The inactive ingredients of Gefitinib tablets are: Tablet core: Lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, povidone, sodium lauryl sulfate and magnesium stearate. Coating: hypromellose, polyethylene glycol 300, titanium dioxide, red ferric oxide and yellow ferric oxide.
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of the clinical antitumor action of gefitinib is not fully characterized. Gefitinib inhibits the intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors, including the tyrosine kinases associated with the epidermal growth factor receptor (EGFR-TK). EGFR is expressed on the cell surface of many normal cells and cancer cells. No clinical studies have been performed that demonstrate a correlation between EGFR receptor expression and response to gefitinib.
Pharmacokinetics
Gefitinib is absorbed slowly after oral administration with mean bioavailability of 60%. Elimination is by metabolism (primarily CYP3A4) and excretion in feces. The elimination half-life is about 48 hours. Daily oral administration of gefitinib to cancer patients resulted in a 2-fold accumulation compared to single dose administration. Steady state plasma concentrations are achieved within 10 days.
Absorption and Distribution
Gefitinib is slowly absorbed, with peak plasma levels occurring 3-7 hours after dosing and mean oral bioavailability of 60%. Bioavailability is not significantly altered by food. Gefitinib is extensively distributed throughout the body with a mean steady state volume of distribution of 1400 L following intravenous administration. In vitro binding of gefitinib to human plasma proteins (serum albumin and α1-acid glycoprotein) is 90% and is independent of drug concentrations.
Metabolism and Elimination
Gefitinib undergoes extensive hepatic metabolism in humans, predominantly by CYP3A4. Three sites of biotransformation have been identified: metabolism of the N-propoxymorpholino-group, demethylation of the methoxy-substituent on the quinazoline, and oxidative defluorination of the halogenated phenyl group.
Five metabolites were identified in human plasma. Only O-desmethyl gefitinib has exposure comparable to gefitinib. Although this metabolite has similar EGFR-TK activity to gefitinib in the isolated enzyme assay, it had only 1/14 of the potency of gefitinib in one of the cell-based assays.
Gefitinib is cleared primarily by the liver, with total plasma clearance and elimination half-life values of 595 mL/min and 48 hours, respectively, after intravenous administration. Excretion is predominantly via the feces (86%), with renal elimination of drug and metabolites accounting for less than 4% of the administered dose.
Special Populations
In population based data analyses, no relationships were identified between predicted steady state trough concentration and patient age, body weight, gender, ethnicity or creatinine clearance.
Pediatric:
There are no pharmacokinetic data in pediatric patients.
Hepatic Impairment:
The influence of hepatic metastases with elevation of serum aspartate aminotransferase (AST/SGOT), alkaline phosphatase, and bilirubin has been evaluated in patients with normal (14 patients), moderately elevated (13 patients) and severely elevated (4 patients) levels of one or more of these biochemical parameters. Patients with moderately and severely elevated biochemical liver abnormalities had gefitinib pharmacokinetics similar to individuals without liver abnormalities.
Renal Impairment:
No clinical studies were conducted with Gefitinib in patients with severely compromised renal function. Gefitinib and its metabolites are not significantly excreted via the kidney (<4%).
Drug-Drug Interactions:
In human liver microsome studies, gefitinib had no inhibitory effect on CYP1A2, CYP2C9, and CYP3A4 activities at concentrations ranging from 2-5000 ng/mL. At the highest concentration studied (5000 ng/mL), gefitinib inhibited CYP2C19 by 24% and CYP2D6 by 43%. Exposure to metoprolol, a substrate of CYP2D6, was increased by 30% when it was given in combination with gefitinib (500 mg daily for 28 days) in patients with solid tumors.
Rifampicin, an inducer of CYP3A4, reduced mean AUC of gefitinib by 85% in healthy male volunteers.
Concomitant administration of itraconazole (200 mg QD for 12 days), an inhibitor of CYP3A4, with gefitinib (250 mg single dose) to healthy male volunteers, increased mean gefitinib AUC by 88%.
Co-administration of high doses of ranitidine with sodium bicarbonate (to maintain the gastric pH above pH 5.0) reduced mean gefitinib AUC by 44%.
International Normalized Ratio (INR) elevations and/or bleeding events have been reported in some patients taking warfarin while on Gefitinib therapy. Patients taking warfarin should be monitored regularly for changes in prothrombin time or INR.
Clinical Studies
Non-Small Cell Lung Cancer (NSCLC):
Refractory Disease Tumor Response Study - A multicenter clinical trial in the United States evaluated the tumor response rate of Gefitinib 250 and 500 mg/day in patients with advanced non-small cell lung cancer whose disease had progressed after at least two prior chemotherapy regimens including a platinum drug and docetaxel. Gefitinib was taken once daily at approximately the same time each day.
Two hundred and sixteen patients received Gefitinib, 102 (47%) and 114 (53%) receiving 250 mg and 500 mg daily doses, respectively. Study patient demographics and disease characteristics are summarized in Table 1. Forty-one percent of the patients had received two prior treatment regimens, 33% three prior treatment regimens, and 25% four or more prior treatment regimens. Effectiveness of Gefitinib as third line therapy was determined in the 142 evaluable patients with documented disease progression on platinum and docetaxel therapies or who had had unacceptable toxicity on these agents.
| Gefitinib Dose | ||
|---|---|---|
| Characteristic | 250 mg/day N = 66 (%) |
500 mg/day N = 76 (%) |
| Age Group | ||
| 18 − 64 years | 43 (65) | 43 (57) |
| 64 − 74 years | 19 (29) | 30 (39) |
| 75 years and above | 4 (6) | 3 (4) |
| Sex | ||
| Male | 38 (58)/td> | 41 (54) |
| Female | 28 (42) | 35 (46) |
| Race | ||
| White | 61 (92) | 68 (89) |
| Black | 1 (2) | 2 (3) |
| Asian/Oriental | 1 (2) | 2 (3) |
| Hispanic | 0 (0) | 3 (4) |
| Other | 3 (5) | 1 (1) |
| Smoking History | ||
| Yes (Previous or current Smoker) | 45 (68) | 62 (82) |
| No (Never Smoked) | 21 (32) | 14 (18) |
| Baseline WHO Performance Status | ||
| 0 | 14 (21) | 9 (12) |
| 1 | 36 (55) | 53 (70) |
| 2 | 15 (23) | 14 (18) |
| Not recorded | 1 (2) | 0 (0) |
| Tumor Histology | ||
| Squamous | 9 (14) | 11 (14) |
| Adenocarcinoma | 47 (71) | 50 (66) |
| Undifferentiated | 6 (9) | 4 (5) |
| Large Cell | 1 (2) | 2 (3) |
| Squamous & Adenocarcinoma | 3 (5) | 7 (9) |
| Not Recorded | 0 (0) | 2 (3) |
| Current Disease Status | ||
| Locally Advanced | 11 (17) | 5 (7) |
| Metastatic | 55 (83) | 71 (93) |
Table 2 shows tumor response rates and response duration. The overall response rate for the 250 and 500 mg doses combined was 10.6% (95% CI: 6%, 16.8%). Response rates appeared to be highly variable in subgroups of the treated population: 5.1% (4/79) in males, 17.5% (11/63) in females, 4.6% (5/108) in previous or current smokers, 29.4% (10/34) in nonsmokers, 12.4% (12/97) with adenocarcinoma histology, and 6.7% (3/45) with other NSCLC histologies. Similar differences were seen in a multinational study in patients who had received 1 or 2 prior chemotherapy regimens, at least 1 of which was platinum-based. In responders, the median time from diagnosis to study randomization was 16.7 months (range 8 to 34 months).
|
|||
| Efficacy Population | |||
|---|---|---|---|
| 250 mg (N=66) | 500 mg (N=76) | Combined (N=142) | |
| Objective Tumor Response Rate (%) | 13.6 | 7.9 | 10.6 |
| 95% CI | 6.4− 24.3 | 3.0 − 16.4 | 6.0 − 16.8 |
| Median Duration of Objective Response (months) | 8.9 | 4.5 | 7.0 |
| Range (months) | 4.6 − 18.6* | 4.4 − 7.6 | 4.4 − 18.6* |
Non-Small Cell Lung Cancer (NSCLC):
Refractory Disease Survival Study - A double-blind, placebo-controlled parallel-group trial randomized 1692 patients with advanced NSCLC to receive either Gefitinib 250 mg daily plus Best Supportive Care or placebo plus Best Supportive Care. Patients had received 1 or 2 prior chemotherapy regimens and had progressed while receiving or within 90 days of the last dose of chemotherapy or were intolerant to the most recent prior chemotherapy regimen. The two treatment arms were well-balanced for demographic and disease-related patient characteristics. The primary endpoint of the study was survival. Gefitinib did not significantly prolong survival (stratified log rank HR 0.89, P=0.11, Median 5.6 vs 5.1 months for Gefitinib and placebo, respectively).
Non-Small Cell Lung Cancer (NSCLC);
Studies of First-line Treatment in Combination with Chemotherapy - Two large trials were conducted in chemotherapy-naïve patients with stage III and IV non-small cell lung cancer. Two thousand one hundred thirty patients were randomized to receive Gefitinib 250 mg daily, Gefitinib 500 mg daily, or placebo in combination with platinum-based chemotherapy regimens. The chemotherapies given in these first-line trials were gemcitabine and cis-platinum (N=1093) or carboplatin and paclitaxel (N=1037). The addition of Gefitinib did not demonstrate any increase, or trend toward such an increase, in tumor response rates, time to progression, or overall survival.
INDICATIONS AND USAGE
Gefitinib is indicated as monotherapy for the continued treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of both platinum-based and docetaxel chemotherapies who are benefiting or have benefited from Gefitinib.
In light of positive survival data with other agents including another oral EGFR inhibitor, physicians should use other treatment options in advanced non-small cell lung cancer patient populations who have received one or two prior chemotherapy regimens and are refractory or intolerant to their most recent regimen.
The effectiveness of Gefitinib was initially based on objective response rates. Subsequent studies intended to demonstrate an increase in survival have been unsuccessful. Specifically, results from a large placebo-controlled randomized trial in patients with advanced NSCLC who progressed while receiving or within 90 days of the last dose of chemotherapy or were intolerant to the most recent prior chemotherapy regimen, did not show an improvement in survival.
Results from two large, controlled, randomized trials in first-line treatment of non-small cell lung cancer showed no benefit from adding Gefitinib to doublet, platinum-based chemotherapy.
CONTRAINDICATIONS
Gefitinib is contraindicated in patients with severe hypersensitivity to gefitinib or to any other component of Gefitinib.
WARNINGS
Pulmonary Toxicity
Cases of interstitial lung disease (ILD) have been observed in patients receiving Gefitinib at an overall incidence of about 1%. Approximately 1/3 of the cases have been fatal. The reported incidence of ILD was about 2% in the Japanese post-marketing experience, about 0.3% in approximately 23,000 patients treated with Gefitinib in a US expanded access program and about 1% in the studies of first-line use in NSCLC (but with similar rates in both treatment and placebo groups).
Reports have described the adverse event as interstitial pneumonia, pneumonitis and alveolitis. Patients often present with the acute onset of dyspnea, sometimes associated with cough or low-grade fever, often becoming severe within a short time and requiring hospitalization. ILD has occurred in patients who have received prior radiation therapy (31% of reported cases), prior chemotherapy (57% of reported patients), and no previous therapy (12% of reported cases). Patients with concurrent idiopathic pulmonary fibrosis whose condition worsens while receiving Gefitinib have been observed to have an increased mortality compared to those without concurrent idiopathic pulmonary fibrosis.
In the event of acute onset or worsening of pulmonary symptoms (dyspnea, cough, fever), Gefitinib therapy should be interrupted and a prompt investigation of these symptoms should occur. If ILD is confirmed, Gefitinib should be discontinued and the patient treated appropriately.
Pregnancy Category D
Gefitinib may cause fetal harm when administered to a pregnant woman. A single dose study in rats showed that gefitinib crosses the placenta after an oral dose of 5 mg/kg (30 mg/m2, about 1/5 the recommended human dose on a mg/m2 basis). When pregnant rats were treated with 5 mg/kg from the beginning of organogenesis to the end of weaning gave birth, there was a reduction in the number of offspring born alive. This effect was more severe at 20 mg/kg and was accompanied by high neonatal mortality soon after parturition. In this study a dose of 1 mg/kg caused no adverse effects.
In rabbits, a dose of 20 mg/kg/day (240 mg/m2, about twice the recommended dose in humans on a mg/m2 basis) caused reduced fetal weight.
There are no adequate and well-controlled studies in pregnant women using Gefitinib. If Gefitinib is used during pregnancy or if the patient becomes pregnant while receiving this drug, she should be apprised of the potential hazard to the fetus or potential risk for loss of the pregnancy.
PRECAUTIONS
General
Hepatotoxicity
Asymptomatic increases in liver transaminases have been observed in Gefitinib treated patients; therefore, periodic liver function (transaminases, bilirubin, and alkaline phosphatase) testing should be considered. Discontinuation of Gefitinib should be considered if changes are severe.
Patients with Hepatic Impairment
In vitro and in vivo evidence suggest that gefitinib is cleared primarily by the liver. Therefore, gefitinib exposure may be increased in patients with hepatic dysfunction. In patients with liver metastases and moderately to severely elevated biochemical liver abnormalities, however, gefitinib pharmacokinetics were similar to the pharmacokinetics of individuals without liver abnormalities. The influence of non-cancer related hepatic impairment on the pharmacokinetics of gefitinib has not been evaluated.
Information for Patients
Patients should be advised to seek medical advice promptly if they develop
- severe or persistent diarrhea, nausea, anorexia, or vomiting, as these have sometimes been associated with dehydration;
- an onset or worsening of pulmonary symptoms, ie, shortness of breath or cough;
- an eye irritation; or,
- any other new symptom.
Women of childbearing potential must be advised to avoid becoming pregnant.
Drug Interactions
Substances that are inducers of CYP3A4 activity increase the metabolism of gefitinib and decrease its plasma concentrations. In patients receiving a potent CYP3A4 inducer such as rifampicin or phenytoin, a dose increase to 500 mg daily should be considered in the absence of severe adverse drug reaction, and clinical response and adverse events should be carefully monitored.
International Normalized Ratio (INR) elevations and/or bleeding events have been reported in some patients taking warfarin while on Gefitinib therapy. Patients taking warfarin should be monitored regularly for changes in prothrombin time or INR.
Substances that are potent inhibitors of CYP3A4 activity (eg, ketoconazole and itraconazole) decrease gefitinib metabolism and increase gefitinib plasma concentrations. This increase may be clinically relevant as adverse experiences are related to dose and exposure; therefore, caution should be used when administering CYP3A4 inhibitors with Gefitinib.
Drugs that cause significant sustained elevation in gastric pH (histamine H2-receptor antagonists such as ranitidine or cimetidine) may reduce plasma concentrations of Gefitinib and therefore potentially may reduce efficacy.
Phase II clinical trial data, where Gefitinib and vinorelbine have been used concomitantly, indicate that Gefitinib may exacerbate the neutropenic effect of vinorelbine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Gefitinib has been tested for genotoxicity in a series of in vitro (bacterial mutation, mouse lymphoma, and human lymphocyte) assays and an in vivo rat micronucleus test. Under the conditions of these assays, gefitinib did not cause genetic damage.
Carcinogenicity studies have not been conducted with gefitinib.
Pregnancy
Pregnancy Category D (see WARNINGS and PRECAUTIONS-Information for Patients sections).
Nursing Mothers
It is not known whether Gefitinib is excreted in human milk. Following oral administration of carbon-14 labeled gefitinib to rats 14 days postpartum, concentrations of radioactivity in milk were higher than in blood. Levels of gefitinib and its metabolites were 11-to-19-fold higher in milk than in blood, after oral exposure of lactating rats to a dose of 5 mg/kg. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, women should be advised against breast-feeding while receiving Gefitinib therapy.
Pediatric Use
Gefitinib is not indicated for use in pediatric patients as safety and effectiveness have not been established (see CLINICAL PHARMACOLOGY-Special Populations-Pediatric).
In clinical trials of Gefitinib alone or with radiation in pediatric patients with primary Central Nervous System (CNS) tumors, cases of CNS hemorrhage and death have been reported. There are insufficient data in pediatric patients to establish a causal relationship. There is no evidence to suggest increased risk of cerebral hemorrhage in adult patients with NSCLC receiving Gefitinib.
Gefitinib is not indicated for use in pediatric patients as safety and effectiveness have not been established.
Geriatric Use
Of the total number of patients participating in trials of second- and third-line Gefitinib treatment of NSCLC, 65% were aged 64 years or less, 30.5 % were aged 65 to 74 years, and 5% of patients were aged 75 years or older. No differences in safety or efficacy were observed between younger and older patients.
Patients with Severe Renal Impairment
The effect of severe renal impairment on the pharmacokinetics of gefitinib is not known. Patients with severe renal impairment should be treated with caution when given Gefitinib.
ADVERSE REACTIONS
The safety database includes 941 patients from clinical trials and approximately 23,000 patients in the Expanded Access Program.
Table 3 includes drug-related adverse events with an incidence of >5% for the 216 patients who received either 250 mg or 500 mg of Gefitinib monotherapy for treatment of NSCLC. The most common adverse events reported at the recommended 250 mg daily dose were diarrhea, rash, acne, dry skin, nausea, and vomiting. The 500 mg dose showed a higher rate for most of these adverse events.
Table 4 provides drug-related adverse events with an incidence of >5% by CTC grade for the patients who received the 250 mg/day dose of Gefitinib monotherapy for treatment of NSCLC. Only 2% of patients stopped therapy due to an adverse drug reaction (ADR). The onset of these ADRs occurred within the first month of therapy.
| Number (%) of Patients | ||
|---|---|---|
| Drug-related adverse event* | 250 mg/day (N=102) % |
500 mg/day (N=114) % |
|
||
| Diarrhea | 49 (48) | 6 (67) |
| Rash | 44 (43) | 61 (54) |
| Acne | 25 (25) | 37 (33) |
| Dry skin | 13 (13) | 30 (26) |
| Nausea | 3 (13) | 20 (18) |
| Vomiting | 12 (12) | 10 (9) |
| Pruritus | (8) | 10 (9) |
| Anorexia | 7 (7) | 11 (10) |
| Asthenia | 6 (6) | 5 (4) |
| Weight loss | 3 (3) | 6 (5) |
| % of Patients | |||||
|---|---|---|---|---|---|
| Adverse Event | All Grades | CTC Grade 1 | CTC Grade 2 | CTC Grade 3 | CTC Grade 4 |
| Diarrhea | 48 | 41 | 6 | 1 | 0 |
| Rash | 43 | 39 | 4 | 0 | 0 |
| Acne | 25 | 19 | 6 | 0 | 0 |
| Dry Skin | 13 | 12 | 1 | 0 | 0 |
| Nausea | 13 | 7 | 5 | 1 | 0 |
| Vomiting | 12 | 9 | 2 | 1 | 0 |
| Pruritus | 8 | 7 | 1 | 0 | 0 |
| Anorexia | 7 | 3 | 4 | 0 | 0 |
| Asthenia | 6 | 2 | 2 | 1 | 1 |
Other adverse events reported at an incidence of <5% in patients who received either 250 mg or 500 mg as monotherapy for treatment of NSCLC (along with their frequency at the 250 mg recommended dose) include the following: peripheral edema (2%), amblyopia (2%), dyspnea (2%), conjunctivitis (1%), vesiculobullous rash (1%), and mouth ulceration (1%).
Interstitial Lung Disease
Cases of interstitial lung disease (ILD) have been observed in patients receiving Gefitinib at an overall incidence of about 1%. Approximately 1/3 of the cases have been fatal. The reported incidence of ILD was about 2% in the Japanese post-marketing experience, about 0.3% in approximately 23,000 patients treated with Gefitinib in a US expanded access program and about 1% in the studies of first-line use in NSCLC (but with similar rates in both treatment and placebo groups).
Reports have described the adverse event as interstitial pneumonia, pneumonitis and alveolitis. Patients often present with the acute onset of dyspnea, sometimes associated with cough or low-grade fever, often becoming severe within a short time and requiring hospitalization. ILD has occurred in patients who have received prior radiation therapy (31% of reported cases), prior chemotherapy (57% of reported patients), and no previous therapy (12% of reported cases). Patients with concurrent idiopathic pulmonary fibrosis whose condition worsens while receiving Gefitinib have been observed to have an increased mortality compared to those without concurrent idiopathic pulmonary fibrosis.
In the event of acute onset or worsening of pulmonary symptoms (dyspnea, cough, fever), Gefitinib therapy should be interrupted and a prompt investigation of these symptoms should occur. If ILD is confirmed, Gefitinib should be discontinued and the patient treated appropriately.
In patients receiving Gefitinib therapy, there were reports of eye pain and corneal erosion/ulcer, sometimes in association with aberrant eyelash growth. Hemorrhage, such as epistaxis and hematuria have been reported in patients receiving Gefitinib. There were also rare reports of pancreatitis and very rare reports of corneal membrane sloughing, ocular ischemia/hemorrhage, toxic epidermal necrolysis, erythema multiforme, and allergic reactions, including angioedema and urticaria.
International Normalized Ratio (INR) elevations and/or bleeding events have been reported in some patients taking warfarin while on Gefitinib therapy. Patients taking warfarin should be monitored regularly for changes in prothrombin time or INR.
Data from non-clinical (in vitro and in vivo) studies indicate that gefitinib has the potential to inhibit the cardiac action potential repolarization process (eg, QT interval). The clinical relevance of these findings is unknown.
OVERDOSAGE
The acute toxicity of gefitinib up to 500 mg in clinical studies has been low. In non-clinical studies, a single dose of 12,000 mg/m2 (about 80 times the recommended clinical dose on a mg/m2 basis) was lethal to rats. Half this dose caused no mortality in mice.
There is no specific treatment for an Gefitinib overdose and possible symptoms of overdose are not established. However, in Phase 1 clinical trials, a limited number of patients were treated with daily doses of up to 1000 mg. An increase in frequency and severity of some adverse reactions was observed, mainly diarrhea and skin rash. Adverse reactions associated with overdose should be treated symptomatically; in particular, severe diarrhea should be managed appropriately.
DOSAGE AND ADMINISTRATION
The recommended daily dose of Gefitinib is one 250 mg tablet with or without food. Higher doses do not give a better response and cause increased toxicity.
For Patients who have Difficulty Swallowing Solids
Gefitinib tablets can also be dispersed in half a glass of drinking water (non-carbonated). No other liquids should be used. Drop the tablet in the water, without crushing it, stir until the tablet is dispersed (approximately 10 minutes) and drink the liquid immediately. Rinse the glass with half a glass of water and drink. The liquid can also be administered through a naso-gastric tube.
Dosage Adjustment
Patients with poorly tolerated diarrhea (sometimes associated with dehydration) or skin adverse drug reactions may be successfully managed by providing a brief (up to 14 days) therapy interruption followed by reinstatement of the 250 mg daily dose.
In the event of acute onset or worsening of pulmonary symptoms (dyspnea, cough, fever), Gefitinib therapy should be interrupted and a prompt investigation of these symptoms should occur and appropriate treatment initiated. If interstitial lung disease is confirmed, Gefitinib should be discontinued and the patient treated appropriately.
Patients who develop onset of new eye symptoms such as pain should be medically evaluated and managed appropriately, including Gefitinib therapy interruption and removal of an aberrant eyelash if present. After symptoms and eye changes have resolved, the decision should be made concerning reinstatement of the 250 mg daily dose.
In patients receiving a potent CYP3A4 inducer such as rifampicin or phenytoin, a dose increase to 500 mg daily should be considered in the absence of severe adverse drug reaction, and clinical response and adverse events should be carefully monitored.
No dosage adjustment is required on the basis of patient age, body weight, gender, ethnicity, or renal function; or in patients with moderate to severe hepatic impairment due to liver metastases.
HOW SUPPLIED
Gefitinib tablets are supplied as round, biconvex, brown film-coated tablets intagliated with "Gefitinib 250" on one side and plain on the other side,each containing 250 mg of gefitinib.
Bottles of 30 Tablets
Storage
Store at controlled room temperature 20-25°C (68-77°F) [see USP].
Gefitinib 250mg Tablets
Description
We are committed to work towards a healthier and happier world. The company is an integrated, research based international pharmaceutical company, producing a wide range of quality, affordable generic (Gefitinib 250mg Tablets) medicines, trusted by health-care professionals and patients across geographies.
We offer you the highest quality new Generic medicines ie. Gefitinib 250mg Tablets, drugs and also with innovative packing at the lowest prices shipped to you from India. Browse our latest Pharmaceuticals and Generics possibilities and other pharmaceuticals possibilities…more.