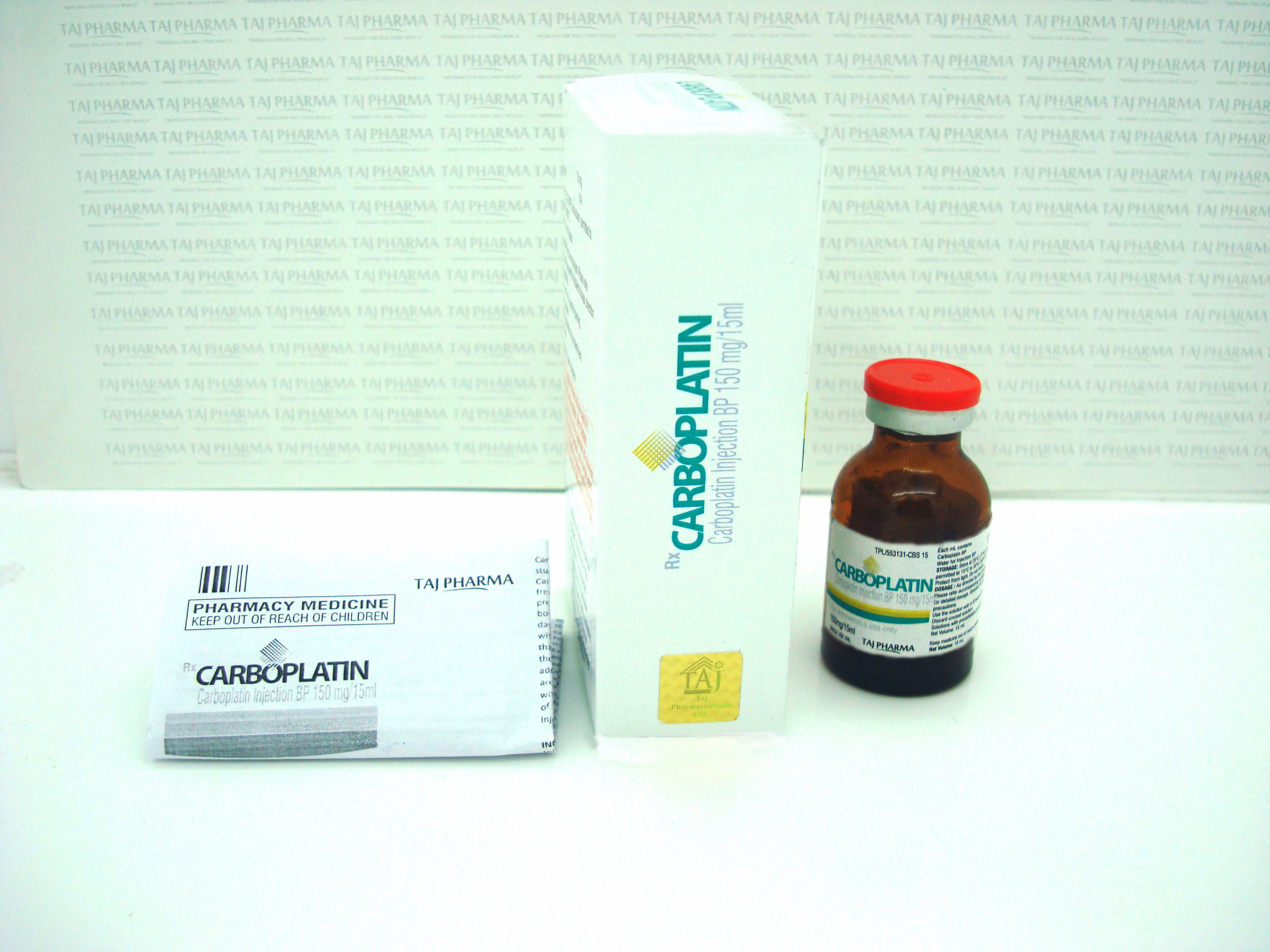
Carboplatin 10mg/50mg Injection
Carboplatin
10mg/50mg Injection
Carboplatin Injection 10 mg/mL sterile solution is available in the following presentations:
50 mg/5 mL vials, individually cartoned. (Blue flip-off seals)
150 mg/15 mL vials, individually cartoned. (Blue flip-off seals)
450 mg/45 mL vials, individually cartoned. (Blue flip-off seals)
600 mg/60 mL vials, individually cartoned. (Blue flip-off seals)
Unopened vials of Carboplatin Injection are stable for the life indicated on the package when stored at 25°C (77°F) [excursions permitted to 15°- 30°C (59°- 86°F) [see USP Controlled Room Temperature] and protected from light.
Carboplatin injection multidose vials maintain microbial, chemical, and physical stability for up to 15 days at 25°C following multiple needle entries.
HANDLING AND DISPOSALProcedures for proper handling and disposal of anti-cancer drugs should be considered. Several guidelines on this subject have been published1-7. There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Aluminum reacts with carboplatin causing precipitate formation and loss of potency, therefore, needles or intravenous sets containing aluminum parts that may come in contact with the drug must not be used for the preparation or administration of Carboplatin Injection.
What Carboplatin looks like and contents of the pack
Carboplatin is a clear, colourless to pale yellow solution free from particles.
Carboplatin is supplied in type I transparent, flint moulded glass vial containing either 5 ml, 15 ml, 45 ml, 60 ml or 100 ml concentrate for solution for infusion. Vials are closed with grey rubber stoppers and white/blue (60 ml vial only) flip-off aluminium seals.
Carboplatin
10mg/50mg Injection
What Carboplatin is and what it is used for
Carboplatin concentrate contains the active ingredient carboplatin which belongs to a group of medicines known as platinum coordination compounds, which is used to treat cancer.
Carboplatin is used against advanced cancer of the ovary and small cell cancer of the lung.
What you need to know before you are given Carboplatin
Do not use Carboplatin:- if you are allergic to carboplatin or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to another drug that belongs to the group of platinum containing compound.
- if you are breast feeding.
- if you have severe problems with your kidney (creatinine clearance below 30 ml/min) and/or liver fuinction.
- if you have an imbalance of your blood cells (severe myelosuppression).
- if you have a tumour that bleeds.
- If you plan to receive a yellow fever vaccination or have just received one.
If any of these apply to you and you have not already discussed this with your doctor or nurse, it is recommended to inform the doctor or nurse as soon as possible and before receiving Carboplatin.
Carboplatin is usually given to patients in hospital. Normally you must not handle this medicine. Your doctor or nurse will administer the medicine and will carefully and frequently monitor you during and after treatment. You will normally have blood tests before each administration.
Warnings and precautionsTalk to your doctor or nurse before using Carboplatin:
- If you are pregnant or there is a chance you may be pregnant.
- If you are breast-feeding.
- If you are likely to drink alcohol whilst treated with this medicine.
- If you are on radiotherapy.
- If you have had or are due to have any vaccination including a live or live-attenuated vaccination.
If your kidneys are not working properly the effects of carboplatin on the blood (haematopoietic system) are increased and prolonged compared to patients with normal kidney function.
In patients with abnormal kidney function visual disturbances, including reversible loss of vision has been reported if carboplatin is used in dose higher than that recommended. The vision may recover totally or to a significant extent upon discontinuation of this medicine.
Your doctor will want to monitor you more regularly if your kidneys are not working properly.
Older people are more likely to experience side effect of the carboplatin on the blood count and nervous system.
If any of these apply to you and you have not already discussed this with your doctor or nurse, it is recommended to inform the doctor or nurse as soon as possible and before receiving the medicine.
This medicine may be diluted with another solution before it is administered. You must discuss this with your doctor and make sure that it is suitable for you.
Talk to your doctor or nurse before being given Carboplatin.
Tell your doctor or nurse if you are using, have recently used or might use any other medicines.
You should tell your doctor if you are taking any of the following medicines as they may interact with Carboplatin:
- yellow fever vaccine and other live vaccines
- other medicines that are known to affect blood cell formation in the bone marrow other medicines that are known to be toxic to your kidney (e.g. aminoglycosides antibiotics, loop diuretics, vancomycin, capreomycin)
- other medicines that are known to damage the hearing or balance functions of the ear (e.g. aminoglycosides antibiotics, furosemide (loop diuretics), [used to treat heart failure and edema], vancomycin, capreomycin)
- other medicines which decrease the activity of the immune system (e.g cyclosporine, tacrolimus, sirolimus and other anticancer medicine)
- blood thinning medicines e.g. warfarin
- phenytoin and fosphenytoin (used to treat various types of convulsions and seizures)
- chelating agents (substances binding to carboplatin thereby decreasing the effect of carboplatin).
There is no known interaction between carboplatin and alcohol. However you must check with your doctor as carboplatin may affect the liver’s ability to cope with alcohol.
Pregnancy, breast-feeding and fertilityIf you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
If any of these apply to you and you have not already discussed this with your doctor or nurse, it is recommended to inform your doctor or nurse as soon as possible and before receiving this medicine.
PregnancyThis medicine must not be used during pregnancy other than in the most exceptional circumstances in the treatment of cancer; where the potential benefit to the mother is considered to be greater than the risk to the unborn baby.
Breast-feedingIf treatment becomes necessary during the lactation period, breastfeeding must be stopped.
FertilityCarboplatin can cause genetic damage. Women are advised to avoid becoming pregnant by using effective contraception before and during treatment. For women who are pregnant or become pregnant during therapy, genetic counselling should be provided.
Men treated with carboplatin are advised not to father a child during, and up to 6 months after treatment. Advice on conservation of sperm should be sought prior to treatment because of the possibility of irreversible infertility.
Ask your doctor or nurse for advice before using any medicine.Driving and using machines
Carboplatin does not affect your ability to drive and use machines. However you should take extra care when you are given carboplatin especially if you are feeling and/or being sick, experiencing visual and hearing disturbances or feeling unsure of yourself.
How to use Carboplatin
Carboplatin will always be administered by a nurse or doctor experienced in the use of cancer treatment. It is usually given in a drip by slow injection into a vein and will usually take between 15 and 60 minutes to be administered.
Dose
Your dose is dependent on your height and weight, function of your blood (haemetopoietic) system and your kidney function. Your doctor will choose the best dose for you. Carboplatin will be diluted before use.
Adults
The usual dose is 400 mg/m2 of your body surface area (calculated from your height and weight).
Older people
For elderly patients (over 65 years old), the dosage may need adjusting depending on your physical condition and laboratory evaluation.
Kidney problems
The amount given may vary, according to how well your kidneys are working. If you suffer from kidney problems, your doctor may reduce the dose and may perform frequent blood tests as well as monitoring your kidney function.
Use in children and adolescents
There has not been enough usage of carboplatin in children to allow the recommendation of specific dose.
You may feel sick while you are being treated with carboplatin. Your doctor may give you another medicine to reduce these effects before you are treated with this medicine.
There will be a usual gap of 4 weeks between each dose of carboplatin. Your doctor will want to perform some blood tests each week after giving you this medicine so he/she can decide on the correct next dosage for you.
It is unlikely that you will be given too much carboplatin. However in the event that this occurs you may have some problems with your kidneys. If you are worried that too much has been administered or you have any questions about the dose being given, you should talk to the doctor administering your medicine.
If you forget to take CarboplatinIt is very unlikely that you will miss a dose of your medicines, as your doctor will have instructions on when to give you your medicine. If you think you have missed your dose please talk to your doctor.
If you stop taking CarboplatinIf you have any further questions on the use of this product ask your doctor or nurse.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you notice any of the following:
- Abnormal bruising, bleeding, or signs of infection such as a sore throat and high temperature.
- Severe itching of the skin (with raised lumps) or swelling of the face, lips, tongue and/or throat, which may cause difficulty in swallowing or breathing (angio-oedema).
- Stomatitis/mucositis (e.g. sore lips or mouth ulcers).
- Changes in your red and white blood cells and platelets (myelosuppression). Your doctor may want to monitor you
- Anaemia (a condition in which there is a decreased number of red blood cells which leads to tiredness)
- Increase in the level of urea and enzymes in your blood. Your doctor may want to monitor you
- Slight loss of hearing
- Abnormal liver function test results. Your doctor may want to monitor you
- Increased uric acid levels in your blood which may lead to gout
- Feeling or being sick
- Abdominal pain or cramp
- Unusual feelings of tiredness or weakness
- Decrease in the level of salts in your blood. Your doctor may want to monitor you Damage to the kidneys (renal toxicity).
- Unusual bruising or bleeding (haemorrhagic complications)
- Reduced function of your kidneys
- Diarrhoea, constipation, sore lips or mouth ulcers (mucositis)
- Allergic reactions including rash, urticaria, skin reddening, itching, high temperature
- Ringing in the ears (tinnitus), hearing impairment and hearing loss
- Pins and needles (peripheral neuropathy)
- Hair loss
- Feeling unwell
- Decreased serum levels of calcium
- Flu-like syndrome
- Loss or lack of body strength
- Fever
- nterstitial lung disease (a group of lung disorders in which the deep lung tissues become inflamed)
- Decrease bone and tendon reflux.
- Secondary malignancies
- Central nervous symptoms often associated with medicine you may be taking to stop you from feeling or being sick
- Fever and chills without evidence of infection
- Redness, swelling and pain or dead skin around the injection site (injection site reaction)
- Infection
- Unusual weight loss
- Change in blood pressure (hyper or hypotension).
- Feeling unwell with a high temperature due to low levels of white blood cells (febrile neutropenia)
- Life threatening infections and bleeding Taste alteration
- Loss of appetite (anorexia)
- Severely impaired liver function, damage or death of liver cells. Your doctor may want to monitor you.
- Temporary visual disturbances including temporary sight loss
- Inflammation of the optic nerve that may cause a complete or partial loss of vision (optic neuritis)
- Haemolytic-uraemic syndrome (a disease characterised by acute renal failure) Decreased number of red blood cells (microangiopathic haemolytic anaemia) and a low platelet count)
- Severe allergic reactions (anaphylaxis/anaphylactic reactions)
- Symptoms of a severe allergic reaction include sudden wheeziness or tightness of chest, swelling of the eyelids, face or lips, facial flushing, hypotension, tachycardia, urticaria, dyspnoea, dizziness and anaphylactic shock.
Very rare (may affect up to 1 in 10,000 people)
Heart failure, blockage in blood vessels of your heart, high blood pressure Bleeding in the brain, which may result in a stroke or loss of consciousness Scarring of the lungs which causes shortness of breath and/or cough (pulmonary fibrosis).
If you get any side effects, talk to your doctor or nurse. This includes any side effects not listed in this leaflet.
Reporting side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects, you can help provide more information on the safety of this medicine.
How to store Carboplatin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial and carton after {EXP}. The expiry date refers to the last day of that month.
Unopened vial: Store below 30°C. Keep the vial in the outer carton in order to protect from light.
Diluted product: Chemical and physical in-use stability has been demonstrated after dilution for 24 hours at 2 to 8oC.
From a microbiological point of view however, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of the user and would normally not be longer than 24 hours at 2 – 8oC, unless dilution has taken place in controlled and validated aseptic conditions.
Contents of the pack and other information
What Carboplatin containsThe active substance is carboplatin.
- One vial of 5 ml contains 50 mg carboplatin.
- One vial of 15 ml contains 150 mg carboplatin.
- One vial of 45 ml contains 450 mg carboplatin.
- One vial of 60 ml contains 600 mg carboplatin.
- One vial of 100 ml contains 1000 mg carboplatin.
The other ingredient is water for injection.
What Carboplatin looks like and contents of the packCarboplatin is a clear, colourless to pale yellow solution free from particles.
Carboplatin is supplied in type I transparent, flint moulded glass vial containing either 5 ml, 15 ml, 45 ml, 60 ml or 100 ml concentrate for solution for infusion. Vials are closed with grey rubber stoppers and white/blue (60 ml vial only) flip-off aluminium seals.
Pack size: 1 vial.
Not all pack sizes may be marketed.
Carboplatin
10mg/50mg Injection
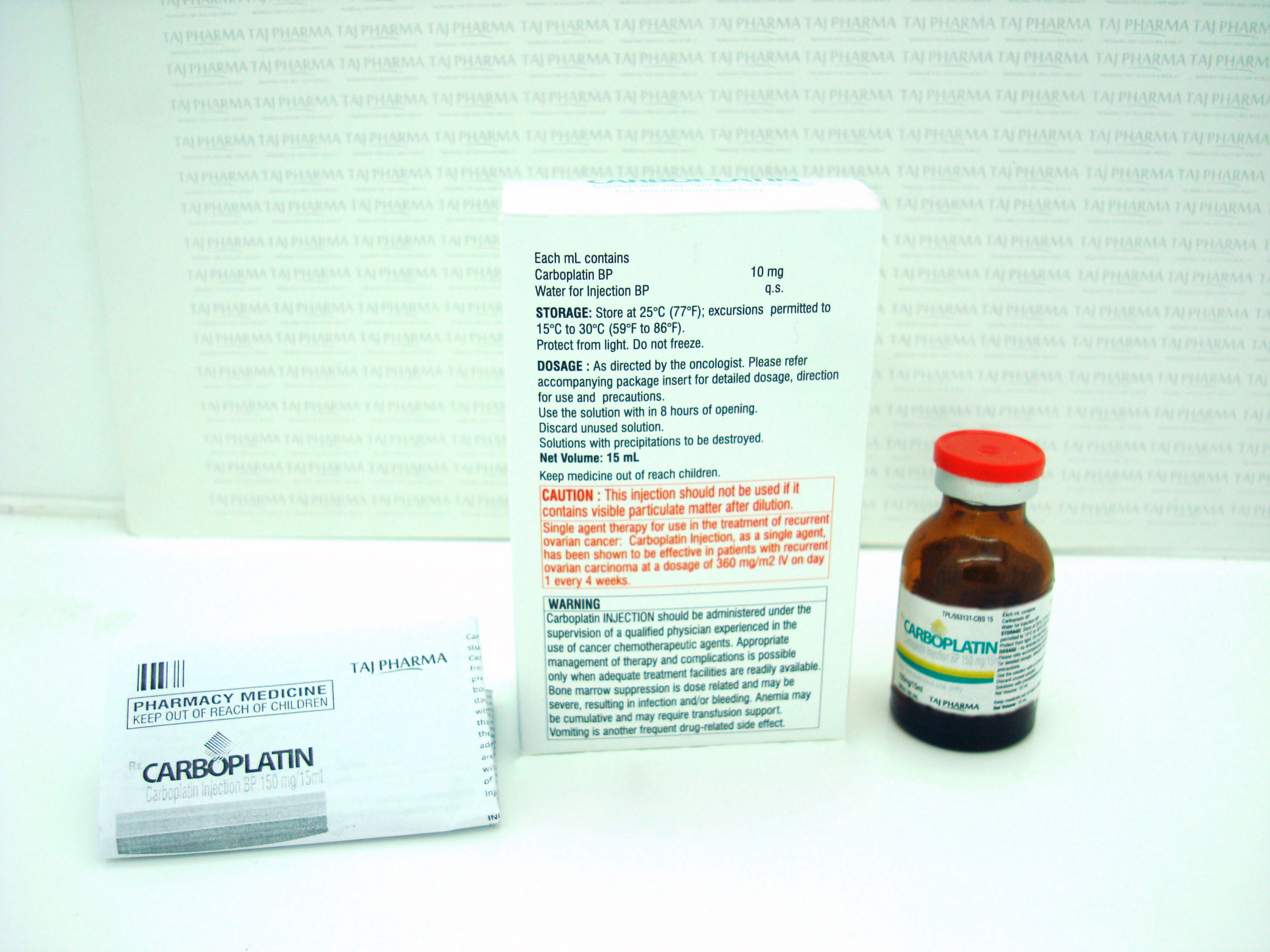
WARNING
Carboplatin Injection should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate treatment facilities are readily available.
Bone marrow suppression is dose related and may be severe, resulting in infection and/or bleeding. Anemia may be cumulative and may require transfusion support. Vomiting is another frequent drug-related side effect.
Anaphylactic-like reactions to Carboplatin Injection have been reported and may occur within minutes of Carboplatin Injection administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms.
DESCRIPTION
Carboplatin Injection is supplied as a sterile, aqueous solution available in 50 mg/5 mL, 150 mg/15 mL, 450 mg/45 mL or 600 mg/60 mL multi-dose vials containing 10 mg/mL of carboplatin for administration by intravenous infusion. Each mL contains 10 mg carboplatin and Water for Injection, USP.
Carboplatin is a platinum coordination compound that is used as a cancer chemotherapeutic agent. The chemical name for carboplatin is platinum, diammine [1,1-cyclobutane-dicarboxylato(2-)-0,0’]-,(SP-4-2), and has the following structural formula:

Carboplatin is a crystalline powder with the molecular formula of C6H12N204Pt and a molecular weight of 371.25. It is soluble in water at a rate of approximately 14 mg/mL, and the pH of a 1% solution is 5-7. It is virtually insoluble in ethanol, acetone, and dimethylacetamide.
CLINICAL PHARMACOLOGY
Carboplatin, like cisplatin, produces predominantly interstrand DNA cross-links rather than DNA -protein cross-links. This effect is apparently cell-cycle nonspecific. The aquation of carboplatin, which is thought to produce the active species, occurs at a slower rate than in the case of cisplatin. Despite this difference, it appears that both carboplatin and cisplatin induce equal numbers of drug-DNA cross-links, causing equivalent lesions and biological effects. The differences in potencies for carboplatin and cisplatin appear to be directly related to the difference in aquation rates.
In patients with creatinine clearances of about 60 mL/min or greater, plasma levels of intact carboplatin decay in a biphasic manner after a 30-minute intravenous infusion of 300 to 500 mg/m2 of Carboplatin Injection. The initial plasma half-life (alpha) was found to be 1.1 to 2 hours (N=6), and the postdistribution plasma half-life (beta) was found to be 2.6 to 5.9 hours (N=6). The total body clearance, apparent volume of distribution and mean residence time for carboplatin are 4.4 L/hour, 16 L and 3.5 hours, respectively. The Cmax values and areas under the plasma concentration vs. time curves from 0 to infinity (AUC inf) increase linearly with dose, although the increase was slightly more than dose proportional. Carboplatin, therefore, exhibits linear pharmacokinetics over the dosing range studied (300 - 500 mg/m2).
Carboplatin is not bound to plasma proteins. No significant quantities of protein-free, ultrafilterable platinum-containing species other than carboplatin are present in plasma. However, platinum from carboplatin becomes irreversibly bound to plasma proteins and is slowly eliminated with a minimum halflife of 5 days.
The major route of elimination of carboplatin is renal excretion. Patients with creatinine clearances of approximately 60 mL/min or greater excrete 65% of the dose in the urine within 12 hours and 71% of the dose within 24 hours. All of the platinum in the 24-hour urine is present as carboplatin. Only 3% to 5% of the administered platinum is excreted in the urine between 24 and 96 hours. There are insufficient data to determine whether biliary excretion occurs.
In patients with creatinine clearances below 60 mL/min the total body and renal clearances of carboplatin decrease as the creatinine clearance decreases. Carboplatin Injection dosages should therefore be reduced in these patients.
The primary determinant of Carboplatin Injection clearance is glomerular filtration rate (GFR) and this parameter of renal function is often decreased in elderly patients. Dosing formulas incorporating estimates of GFR to provide predictable Carboplatin Injection plasma AUCs should be used in elderly patients to minimize the risk of toxicity.
CLINICAL STUDIES
Use with Cyclophosphamide for Initial Treatment of Ovarian Cancer:
In two prospectively randomized, controlled studies conducted by the National Cancer Institute of Canada, Clinical Trials Group (NCIC) and the Southwest Oncology Group (SWOG), 789 chemotherapy naive patients with advanced ovarian cancer were treated with Carboplatin Injection or cisplatin, both in combination with cyclophosphamide every 28 days for six courses before surgical reevaluation. The following results were obtained from both studies:
| Comparative Efficacy: | ||
|---|---|---|
| Overview of Pivotal Trials | ||
| NCIC | SWOG | |
| *114 Carboplatin and 109 Cisplatin patients did not undergo second look surgery in NCIC study. 90 Carboplatin and 106 Cisplatin patients did not undergo second look surgery in SWOG study. | ||
| Number of patients randomized | 447 | 342 |
| Median age (years) | 60 | 62 |
| Dose of cisplatin | 75 mg/m2 | 100 mg/m2 |
| Dose of carboplatin | 300 mg/m2 | 300 mg/m2 |
| Dose of cyclophosphamide | 600 mg/m2 | 600 mg/m2 |
| Residual tumor <2 cm (number of patients) | 39% (174/447) | 14% (49/342) |
| Clinical Response in Measurable Disease Patients | ||
| NCIC | SWOG | |
| Carboplatin (number of patients) | 60% (48/80) | 58% (48/83) |
| Cisplatin (number of patients) | 58% (49/85) | 43% (33/76) |
| 95% C.I. of difference (Carboplatin - Cisplatin) | (-13.9%, 18.6%) | (-2.3%, 31.1%) |
| Pathologic Complete Response* | ||
| NCIC | SWOG | |
| Carboplatin (number of patients) | 11% (24/224) | 10% (17/171) |
| Cisplatin (number of patients) | 15% (33/223) | 10% (17/171) |
| 95% C.I. of difference (Carboplatin - Cisplatin) | (-10.7%, 2.5%) | (-6.9%, 6.9%) |
| NCIC | SWOG | |
|---|---|---|
| *Kaplan-Meier Estimates Unrelated deaths occurring in the absence of progression were counted as events (progression) in this analysis. | ||
| ** Analysis adjusted for factors found to be of prognostic significance were consistent with unadjusted analysis. | ||
| Median | ||
| Carboplatin | 59 weeks | 49 weeks |
| Cisplatin | 61 weeks | 47 weeks |
| 2-Year PFS* | ||
| Carboplatin | 31% | 21% |
| Cisplatin | 31% | 21% |
| 95% C.I. of difference (Carboplatin - Cisplatin) | (-9.3, 8.7) | (-9.0, 9.4) |
| 3-Year PFS* | ||
| Carboplatin | 19% | 8% |
| Cisplatin | 23% | 14% |
| 95% C.I. of difference (Carboplatin - Cisplatin) | (-11.5, 4.5) | (-14.1, 0.3) |
| Hazard ratio** | 1.10 | 1.02 |
| 95% C.I. (Carboplatin - Cisplatin) | (0.89, 1.35) | (0.81, 1.29) |
| NCIC | SWOG | |
|---|---|---|
| *Kaplan-Meier Estimates | ||
| **Analysis adjusted for factors found to be of prognostic significance were consistent with unadjusted analysis. | ||
| Median | ||
| Carboplatin | 110 weeks | 86 weeks |
| Cisplatin | 99 weeks | 79 weeks |
| 2-Year Survival*> | ||
| Carboplatin | 51.9% | 40.2% |
| Cisplatin | 48.4% | 39.0% |
| 95% C.I. of difference (Carboplatin - Cisplatin) | (-6.2, 13.2) | (-9.8, 12.2) |
| 3-Year Survival* | ||
| Carboplatin | 34.6% | 18.3% |
| Cisplatin | 33.1% | 24.9% |
| 95% C.I. of difference (Carboplatin - Cisplatin) | (-7.7, 10.7) | (-15.9, 2.7) |
| Hazard Ratio** | 0.98 | 1.01 |
| 95% C.I. (Carboplatin - Cisplatin) | (0.78, 1.23) | (0.78, 1.30) |
Comparative Toxicity:
The pattern of toxicity exerted by the Carboplatin Injection-containing regimen was significantly different from that of the cisplatin-containing combinations. Differences between the two studies may be explained by different cisplatin dosages and by different supportive care.
The Carboplatin Injection-containing regimen induced significantly more thrombocytopenia and, in one study, significantly more leukopenia and more need for transfusional support. The cisplatin-containing regimen produced significantly more anemia in one study. However, no significant differences occurred in incidences of infections and hemorrhagic episodes.
Non-hematologic toxicities (emesis, neurotoxicity, ototoxicity, renal toxicity, hypomagnesemia, and alopecia) were significantly more frequent in the cisplatin-containing arms.
| Carboplatin Arm Percent* | Cisplatin Arm Percent* | P-Values** | ||
|---|---|---|---|---|
| *Values are in percent of evaluable patients | ||||
| **n.s. = not significant, p>0.05 | ||||
| +May have been affected by cyclophosphamide dosage delivered | ||||
| Bone Marrow | ||||
| Thrombocytopenia | <100,000/mm3 | 70 | 29 | <0.001 |
| <50,000/mm3 | 41 | 6 | <0.001 | |
| Neutropenia | <2000 cells/mm3 | 97 | 96 | n.s. |
| <1000 cells/mm3 | 81 | 79 | n.s. | |
| Leukopenia | <4000 cells/mm3 | 98 | 97 | n.s. |
| <2000 cells/mm3 | 68 | 52 | 0.001 | |
| Anemia | <11 g/dL | 91 | 91 | n.s. |
| <8 g/dL | 18 | 12 | n.s. | |
| Infections | 14 | 12 | n.s. | |
| Bleeding | 10 | 4 | n.s. | |
| Transfusions | 42 | 31 | 0.018 | |
| Gastrointestinal | ||||
| Nausea and vomiting | 93 | 98 | 0.010 | |
| Vomiting | 84 | 97 | <0.001 | |
| Other GI side effects | 50 | 62 | 0.013 | |
| Neurologic | ||||
| Peripheral neuropathies | 16 | 42 | <0.001 | |
| Ototoxicity | 13 | 33 | <0.001 | |
| Other sensory side effects | 6 | 10 | n.s. | |
| Central neurotoxicity | 28 | 40 | 0.009 | |
| Renal | ||||
| Serum creatinine elevations | 5 | 13 | 0.006 | |
| Blood urea elevations | 17 | 31 | <0.001 | |
| Hepatic | ||||
| Bilirubin elevations | 5 | 3 | n.s. | |
| SGOT elevations | 17 | 13 | n.s. | |
| Alkaline phosphatase elevations | - | - | - | |
| Electrolytes loss | ||||
| Sodium | 10 | 20 | 0.005 | |
| Potassium | 16 | 22 | n.s. | |
| Calcium | 16 | 19 | n.s. | |
| Magnesium | 63 | 88 | <0.001 | |
| Other side effects | ||||
| Pain | 36 | 37 | n.s. | |
| Asthenia | 40 | 33 | n.s. | |
| Cardiovascular | 15 | 19 | n.s. | |
| Respiratory | 8 | 9 | n.s. | |
| Allergic | 12 | 9 | n.s. | |
| Genitourinary | 10 | 10 | n.s. | |
| Alopecia + | 50 | 62 | 0.017 | |
| Mucositis | 10 | 9 | n.s. | |
| Carboplatin Arm Percent* | Cisplatin Arm Percent* | P-Values** | ||
|---|---|---|---|---|
| *Values are in percent of evaluable patients | ||||
| **n.s. = not significant, p>0.05 | ||||
| +May have been affected by cyclophosphamide dosage delivered | ||||
| Bone Marrow | ||||
| Thrombocytopenia | <100,000/mm3 | 59 | 35 | <0.001 |
| <50,000/mm3 | 22 | 11 | 0.006 | |
| Neutropenia | <2000 cells/mm3 | 95 | 97 | n.s. |
| <1000 cells/mm3 | 84 | 78 | n.s. | |
| Leukopenia | <4000 cells/mm3 | 97 | 97 | n.s. |
| <2000 cells/mm3 | 76 | 67 | n.s. | |
| Anemia | <11 g/dL | 88 | 87 | n.s. |
| <8 g/dL | 8 | 24 | <0.001 | |
| Infections | 18 | 21 | n.s. | |
| Bleeding | 6 | 4 | n.s. | |
| Transfusions | 25 | 33 | n.s. | |
| Gastrointestinal | ||||
| Nausea and vomiting | 94 | 96 | n.s. | |
| Vomiting | 82 | 91 | 0.007 | |
| Other GI side effects | 40 | 48 | n.s. | |
| Neurologic | ||||
| Peripheral neuropathies | 13 | 28 | 0.001 | |
| Ototoxicity | 12 | 30 | <0.001 | |
| Other sensory side effects | 4 | 6 | n.s. | |
| Central neurotoxicity | 23 | 29 | n.s. | |
| Renal | ||||
| Serum creatinine elevations | 7 | 38 | <0.001 | |
| Blood urea elevations | - | - | - | |
| Hepatic | ||||
| Bilirubin elevations | 5 | 3 | n.s. | |
| SGOT elevations | 23 | 16 | n.s. | |
| Alkaline phosphatase elevations | 29 | 20 | n.s. | |
| Electrolytes loss | ||||
| Sodium | - | - | - | |
| Potassium | - | - | - | |
| Calcium | - | - | - | |
| Magnesium | 58 | 77 | <0.001 | |
| Other side effects | ||||
| Pain | 54 | 52 | n.s. | |
| Asthenia | 43 | 46 | n.s. | |
| Cardiovascular | 23 | 30 | n.s. | |
| Respiratory | 12 | 11 | n.s. | |
| Allergic | 10 | 11 | n.s. | |
| Genitourinary | 11 | 13 | n.s. | |
| Alopecia + | 43 | 57 | 0.009 | |
| Mucositis | 6 | 11 | n.s. | |
Use as a Single Agent for Secondary Treatment of Advanced Ovarian Cancer:
In two prospective, randomized controlled studies in patients with advanced ovarian cancer previously treated with chemotherapy, Carboplatin Injection achieved six clinical complete responses in 47 patients. The duration of these responses ranged from 45 to 71 + weeks.
INDICATIONS AND USAGE
Initial Treatment of Advanced Ovarian Carcinoma:
Carboplatin Injection is indicated for the initial treatment of advanced ovarian carcinoma in established combination with other approved chemotherapeutic agents. One established combination regimen consists of Carboplatin Injection and cyclophosphamide. Two randomized controlled studies conducted by the NCIC and SWOG with carboplatin vs. cisplatin, both in combination with cyclophosphamide, have demonstrated equivalent overall survival between the two groups.
There is limited statistical power to demonstrate equivalence in overall pathologic complete response rates and long-term survival (≥3 years) because of the small number of patients with these outcomes: the small number of patients with residual tumor <2 cm after initial surgery also limits the statistical power to demonstrate equivalence in this subgroup.
Secondary Treatment of Advanced Ovarian Carcinoma:
Carboplatin Injection is indicated for the palliative treatment of patients with ovarian carcinoma recurrent after prior chemotherapy, including patients who have been previously treated with cisplatin.
Within the group of patients previously treated with cisplatin, those who have developed progressive disease while receiving cisplatin therapy may have a decreased response rate.
CONTRAINDICATIONS
Carboplatin Injection is contraindicated in patients with a history of severe allergic reactions to cisplatin or other platinum-containing compounds.
Carboplatin Injection should not be employed in patients with severe bone marrow depression or significant bleeding.
WARNINGS
Bone marrow suppression (leukopenia, neutropenia, and thrombocytopenia) is dose-dependent and is also the dose-limiting toxicity. Peripheral blood counts should be frequently monitored during carboplatin treatment and, when appropriate, until recovery is achieved. Median nadir occurs at day 21 in patients receiving single agent carboplatin. In general, single intermittent courses of carboplatin should not be repeated until leukocyte, neutrophil, and platelet counts have recovered.
Since anemia is cumulative, transfusions may be needed during treatment with carboplatin, particularly in patients receiving prolonged therapy.
Bone marrow suppression is increased in patients who have received prior therapy, especially regimens including cisplatin. Marrow suppression is also increased in patients with impaired kidney function. Initial carboplatin dosages in these patients should be appropriately reduced and blood counts should be carefully monitored between courses. The use of carboplatin in combination with other bone marrow suppressing therapies must be carefully managed with respect to dosage and timing in order to minimize additive effects.
Carboplatin has limited nephrotoxic potential, but concomitant treatment with aminoglycosides has resulted in increased renal and/or audiologic toxicity, and caution must be exercised when a patient receives both drugs. Clinically significant hearing loss has been reported to occur in pediatric patients when carboplatin was administered at higher than recommended doses in combination with other ototoxic agents.
Carboplatin can induce emesis, which can be more severe in patients previously receiving emetogenic therapy. The incidence and intensity of emesis have been reduced by using premedication with antiemetics. Although no conclusive efficacy data exist with the following schedules of carboplatin, lengthening the duration of single intravenous administration to 24 hours or dividing the total dose over five consecutive daily pulse doses has resulted in reduced emesis.
Although peripheral neurotoxicity is infrequent, its incidence is increased in patients older than 65 years and in patients previously treated with cisplatin. Pre-existing cisplatin-induced neurotoxicity does not worsen in about 70% of the patients receiving carboplatin as secondary treatment.
Loss of vision, which can be complete for light and colors, has been reported after the use of carboplatin with doses higher than those recommended in the package insert. Vision appears to recover totally or to a significant extent within weeks of stopping these high doses.
As in the case of other platinum-coordination compounds, allergic reactions to carboplatin have been reported. These may occur within minutes of administration and should be managed with appropriate supportive therapy. There is increased risk of allergic reactions including anaphylaxis in patients previously exposed to platinum therapy.
High dosages of carboplatin (more than four times the recommended dose) have resulted in severe abnormalities of liver function tests.
Carboplatin may cause fetal harm when administered to a pregnant woman. Carboplatin has been shown to be embryotoxic and teratogenic in rats. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
PRECAUTIONS
General:Needles or intravenous administration sets containing aluminum parts that may come in contact with carboplatin should not be used for the preparation or administration of the drug. Aluminum can react with carboplatin causing precipitate formation and loss of potency.
Drug Interactions:The renal effects of nephrotoxic compounds may be potentiated by carboplatin.
Carcinogenesis, Mutagenesis, Impairment of Fertility:The carcinogenic potential of carboplatin has not been studied, but compounds with similar mechanisms of action and mutagenicity profiles have been reported to be carcinogenic. Carboplatin has been shown to be mutagenic both in vitro and in vivo. It has also been shown to be embryotoxic and teratogenic in rats receiving the drug during organogenesis. Secondary malignancies have been reported in association with multi-drug therapy.
Pregnancy:Pregnancy “Category D” (see WARNINGS).
Nursing Mothers:It is not known whether carboplatin is excreted in human milk. Because there is a possibility of toxicity in nursing infants secondary to carboplatin treatment of the mother, it is recommended that breast feeding be discontinued if the mother is treated with carboplatin.
Pediatric Use:Safety and effectiveness in pediatric patients have not been established (see WARNINGS; “Audiologic Toxicity”).
Geriatric Use:Of the 789 patients in initial treatment combination therapy studies (NCIC and SWOG), 395 patients were treated with carboplatin in combination with cyclophosphamide. Of these, 141 were over 65 years of age and 22 were 75 years or older. In these trials, age was not a prognostic factor for survival. In terms of safety, elderly patients treated with carboplatin were more likely to develop severe thrombocytopenia than younger patients. In a combined database of 1942 patients (414 were ≥65 years of age) that received single agent carboplatin for different tumor types, a similar incidence of adverse events was seen in patients 65 years and older and in patients less than 65. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Because renal function is often decreased in the elderly, renal function should be considered in the selection of carboplatin dosage.
ADVERSE REACTIONS
For a comparison of toxicities when carboplatin or cisplatin was given in combination with cyclophosphamide, see the Comparative Toxicity subsection of the CLINICAL STUDIES section.
| First Line Combination Therapy* Percent | Second Line Single Agent Therapy** Percent | ||
|---|---|---|---|
* Use with Cyclophosphamide for Initial Treatment of Ovarian Cancer: Data are based on the experience of 393 patients with ovarian cancer (regardless of baseline status) who received initial combination therapy with carboplatin and cyclophosphamide in two randomized controlled studies conducted by SWOG and NCIC (see CLINICAL STUDIES). Combination with cyclophosphamide as well as duration of treatment may be responsible for the differences that can be noted in the adverse experience table. |
|||
| ** Single Agent Use for the Secondary Treatment of Ovarian Cancer: Data are based on the experience of 553 patients with previously treated ovarian carcinoma (regardless of baseline status) who received single-agent carboplatin. | |||
| Bone Marrow | |||
| Thrombocytopenia | <100,000/mm3 | 66 | 62 |
| <50,000/mm3 | 33 | 35 | |
| Neutropenia | <2000 cells/mm3 | 96 | 67 |
| <1000 cells/mm3 | 82 | 21 | |
| Leukopenia | <4000 cells/mm3 | 97 | 85 |
| <2000 cells/mm3 | 71 | 26 | |
| Anemia | <11 g/dL | 90 | 90 |
| <8 g/dL | 14 | 21 | |
| Infections | 16 | 5 | |
| Bleeding | 8 | 5 | |
| Transfusions | 35 | 44 | |
| Gastrointestinal | |||
| Nausea and vomiting | 93 | 92 | |
| Vomiting | 83 | 81 | |
| Other GI side effects | 46 | 21 | |
| Neurologic | |||
| Peripheral neuropathies | 15 | 6 | |
| Ototoxicity | 12 | 1 | |
| Other sensory side effects | 5 | 1 | |
| Central neurotoxicity | 26 | 5 | |
| Renal | |||
| Serum creatinine elevations | 6 | 10 | |
| Blood urea elevations | 17 | 22 | |
| Hepatic | |||
| Bilirubin elevations | 5 | 5 | |
| SGOT elevations | 20 | 19 | |
| Alkaline phosphatase elevations | 29 | 37 | |
| Electrolytes loss | |||
| Sodium | 10 | 47 | |
| Potassium | 16 | 28 | |
| Calcium | 16 | 31 | |
| Magnesium | 61 | 43 | |
| Other side effects | |||
| Pain | 44 | 23 | |
| Asthenia | 41 | 11 | |
| Cardiovascular | 19 | 6 | |
| Respiratory | 10 | 6 | |
| Allergic | 11 | 2 | |
| Genitourinary | 10 | 2 | |
| Alopecia | 49 | 2 | |
| Mucositis | 8 | 1 | |
In the narrative section that follows, the incidences of adverse events are based on data from 1893 patients with various types of tumors who received carboplatin as single-agent therapy.
Hematologic Toxicity:
Bone marrow suppression is the dose-limiting toxicity of carboplatin. Thrombocytopenia with platelet counts below 50,000/mm3 occurs in 25% of the patients (35% of pretreated ovarian cancer patients); neutropenia with granulocyte counts below 1,000/mm3 occurs in 16% of the patients (21% of pretreated ovarian cancer patients); leukopenia with WBC counts below 2,000/mm3 occurs in 15% of the patients (26% of pretreated ovarian cancer patients). The nadir usually occurs about day 21 in patients receiving single-agent therapy. By day 28, 90% of patients have platelet counts above 100,000/mm3; 74% have neutrophil counts above 2,000/mm3; 67% have leukocyte counts above 4,000/mm3.
Marrow suppression is usually more severe in patients with impaired kidney function. Patients with poor performance status have also experienced a higher incidence of severe leukopenia and thrombocytopenia.
The hematologic effects, although usually reversible, have resulted in infectious or hemorrhagic complications in 5% of the patients treated with carboplatin, with drug related death occurring in less than 1% of the patients. Fever has also been reported in patients with neutropenia.
Anemia with hemoglobin less than 11 g/dL has been observed in 71% of the patients who started therapy with a baseline above that value. The incidence of anemia increases with increasing exposure to carboplatin. Transfusions have been administered to 26% of the patients treated with carboplatin (44% of previously treated ovarian cancer patients).
Bone marrow depression may be more severe when carboplatin is combined with other bone marrow suppressing drugs or with radiotherapy.
Gastrointestinal Toxicity:
Vomiting occurs in 65% of the patients (81% of previously treated ovarian cancer patients) and in about one-third of these patients it is severe. Carboplatin, as a single agent or in combination, is significantly less emetogenic than cisplatin; however, patients previously treated with emetogenic agents, especially cisplatin, appear to be more prone to vomiting. Nausea alone occurs in an additional 10% to 15% of patients. Both nausea and vomiting usually cease within 24 hours of treatment and are often responsive to antiemetic measures. Although no conclusive efficacy data exist with the following schedules, prolonged administration of carboplatin, either by continuous 24-hour infusion or by daily pulse doses given for five consecutive days, was associated with less severe vomiting than the single dose intermittent schedule. Emesis was increased when carboplatin was used in combination with other emetogenic compounds. Other gastrointestinal effects observed frequently were pain, in 17% of the patients; diarrhea, in 6%; and constipation, also in 6%.
Neurologic Toxicity:
Peripheral neuropathies have been observed in 4% of the patients receiving carboplatin (6% of pretreated ovarian cancer patients) with mild paresthesias occurring most frequently. Carboplatin therapy produces significantly fewer and less severe neurologic side effects than does therapy with cisplatin. However, patients older than 65 years and/or previously treated with cisplatin appear to have an increased risk (10%) for peripheral neuropathies. In 70% of the patients with pre-existing cisplatin-induced peripheral neurotoxicity, there was no worsening of symptoms during therapy with carboplatin. Clinical ototoxicity and other sensory abnormalities such as visual disturbances and change in taste have been reported in only 1% of the patients. Central nervous system symptoms have been reported in 5% of the patients and appear to be most often related to the use of antiemetics.
Although the overall incidence of peripheral neurologic side effects induced by carboplatin is low, prolonged treatment, particularly in cisplatin pretreated patients, may result in cumulative neurotoxicity.
Nephrotoxicity:
Development of abnormal renal function test results is uncommon, despite the fact that carboplatin, unlike cisplatin, has usually been administered without high-volume fluid hydration and/or forced diuresis. The incidences of abnormal renal function tests reported are 6% for serum creatinine and 14% for blood urea nitrogen (10% and 22%, respectively, in pretreated ovarian cancer patients). Most of these reported abnormalities have been mild and about one-half of them were reversible.
Creatinine clearance has proven to be the most sensitive measure of kidney function in patients receiving carboplatin, and it appears to be the most useful test for correlating drug clearance and bone marrow suppression. Twenty-seven percent of the patients who had a baseline value of 60 mL/min or more demonstrated a reduction below this value during carboplatin therapy.
Hepatic Toxicity:
The incidences of abnormal liver function tests in patients with normal baseline values were reported as follows: total bilirubin, 5%; SGOT, 15%; and alkaline phosphatase, 24%; (5%, 19%, and 37%, respectively, in pretreated ovarian cancer patients). These abnormalities have generally been mild and reversible in about one-half of the cases, although the role of metastatic tumor in the liver may complicate the assessment in many patients. In a limited series of patients receiving very high dosages of carboplatin and autologous bone marrow transplantation, severe abnormalities of liver function tests were reported.
Electrolyte Changes:
The incidences of abnormally decreased serum electrolyte values reported were as follows: sodium, 29%; potassium, 20%; calcium, 22%; and magnesium, 29%; (47%, 28%, 31%, and 43%, respectively, in pretreated ovarian cancer patients). Electrolyte supplementation was not routinely administered concomitantly with carboplatin, and these electrolyte abnormalities were rarely associated with symptoms.
Allergic Reactions:
Hypersensitivity to carboplatin has been reported in 2% of the patients. These allergic reactions have been similar in nature and severity to those reported with other platinum-containing compounds, i.e., rash, urticaria, erythema, pruritus, and rarely bronchospasm and hypotension. Anaphylactic reactions have been reported as part of post-marketing surveillance. These reactions have been successfully managed with standard epinephrine, corticosteroid, and antihistamine therapy.
Injection Site Reactions:
Injection site reactions, including redness, swelling, and pain, have been reported during postmarketing surveillance. Necrosis associated with extravasation has also been reported.
Other Events:
Pain and asthenia were the most frequently reported miscellaneous adverse effects; their relationship to the tumor and to anemia was likely. Alopecia was reported (3%). Cardiovascular, respiratory, genitourinary, and mucosal side effects have occurred in 6% or less of the patients. Cardiovascular events (cardiac failure, embolism, cerebrovascular accidents) were fatal in less than 1% of the patients and did not appear to be related to chemotherapy. Cancer-associated hemolytic uremic syndrome has been reported rarely.
Malaise, anorexia and hypertension have been reported as part of post marketing surveillance.
OVERDOSAGE
There is no known antidote for Carboplatin Injection overdosage. The anticipated complications of overdosage would be secondary to bone marrow suppression and/or hepatic toxicity.
DOSAGE AND ADMINISTRATION
NOTE: Aluminum reacts with carboplatin causing precipitate formation and loss of potency, therefore, needles or intravenous sets containing aluminum parts that may come in contact with the drug must not be used for the preparation or administration of Carboplatin Injection.
Single Agent Therapy:
Carboplatin Injection, as a single agent, has been shown to be effective in patients with recurrent ovarian carcinoma at a dosage of 360 mg/m2 IV on day 1 every 4 weeks (alternatively see Formula Dosing). In general, however, single intermittent courses of Carboplatin Injection should not be repeated until the neutrophil count is at least 2000 and the platelet count is at least 100,000.
Combination Therapy with Cyclophosphamide:
In the chemotherapy of advanced ovarian cancer, an effective combination for previously untreated patients consists of:
Carboplatin Injection - 300 mg/m2 IV on day 1 every four weeks for six cycles (alternatively see Formula Dosing).
Cyclophosphamide - 600 mg/m2 IV on day 1 every four weeks for six cycles. For directions regarding the use and administration of cyclophosphamide please refer to its package insert. (See CLINICAL STUDIES.)
Intermittent courses of Carboplatin Injection in combination with cyclophosphamide should not be repeated until the neutrophil count is at least 2000 and the platelet count is at least 100,000.
Dose Adjustment Recommendations:
Pretreatment platelet count and performance status are important prognostic factors for severity of myelosuppression in previously treated patients.
The suggested dose adjustments for single agent or combination therapy shown in the table below are modified from controlled trials in previously treated and untreated patients with ovarian carcinoma. Blood counts were done weekly, and the recommendations are based on the lowest post-treatment platelet or neutrophil value.
| Platelets | Neutrophils | Adjusted Dose* (From Prior Course) |
|---|---|---|
| *Percentages apply to Carboplatin Injection as a single agent or to both Carboplatin Injection and cyclophosphamide in combination. In the controlled studies, dosages were also adjusted at a lower level (50% to 60%) for severe myelosuppression. Escalations above 125% were not recommended for these studies. | ||
| >100,000 | >2000 | 125% |
| 50-100,000 | 500-2000 | No Adjustment |
| <50,000 | <500 | 75% |
Carboplatin Injection is usually administered by an infusion lasting 15 minutes or longer. No pre- or post-treatment hydration or forced diuresis is required.
Patients with Impaired Kidney Function:
Patients with creatinine clearance values below 60 mL/min are at increased risk of severe bone marrow suppression. In renally-impaired patients who received single-agent Carboplatin Injection therapy, the incidence of severe leukopenia, neutropenia, or thrombocytopenia has been about 25% when the dosage modifications in the table below have been used.
| Baseline Creatinine Clearance | Recommended Dose on Day 1 |
|---|---|
| 41 - 59 mL/min | 250 mg/m2 |
| 16 - 40 mL/min | 200 mg/m2 |
The data available for patients with severely impaired kidney function (creatinine clearance below 15 mL/min) are too limited to permit a recommendation for treatment.
These dosing recommendations apply to the initial course of treatment. Subsequent dosages should be adjusted according to the patient’s tolerance based on the degree of bone marrow suppression.
Formula Dosing:
Another approach for determining the initial dose of Carboplatin Injection is the use of mathematical formulae, which are based on a patient’s preexisting renal function or renal function and desired platelet nadir. Renal excretion is the major route of elimination for carboplatin. The use of dosing formulae, as compared to empirical dose calculation based on body surface area, allows compensation for patient variations in pretreatment renal function that might otherwise result in either underdosing (in patients with above average renal function) or overdosing (in patients with impaired renal function).
A simple formula for calculating dosage, based upon a patient’s glomerular filtration rate (GFR in mL/min) and Carboplatin Injection target area under the concentration versus time curve (AUC in mg/mL•min), has been proposed by Calvert. In these studies, GFR was measured by 51Cr-EDTA clearance.
| Total Dose (mg) = (target AUC) x (GFR + 25) |
|---|
| Note: With the Calvert formula, the total dose of Carboplatin Injection is calculated in mg, not mg/m2. |
The target AUC of 4-6 mg/mL•min using single agent Carboplatin Injection appears to provide the most appropriate dose range in previously treated patients. This study also showed a trend between the AUC of single agent Carboplatin Injection administered to previously treated patients and the likelihood of developing toxicity.
| % Actual Toxicity in Previously Treated Patients | ||
|---|---|---|
| Gr 3 or Gr 4 | Gr 3 or Gr 4 | |
| AUC (mg/mL•min) | Thrombocytopenia | Leukopenia |
| 4 to 5 | 16% | 13% |
| 6 to 7 | 33 | 34% |
Geriatric Dosing:
Because renal function is often decreased in elderly patients, formula dosing of Carboplatin Injection based on estimates of GFR should be used in elderly patients to provide predictable plasma Carboplatin Injection AUCs and thereby minimize the risk of toxicity.
PREPARATION OF INTRAVENOUS SOLUTIONS
Carboplatin Injection 10 mg/mL is supplied as a Ready To Use (RTU) sterile solution in 5 mL, 15 mL, 45 mL or 60 mL vials. Total content of carboplatin per vial is described in following table:
| Vial Strength | Diluent Volume |
|---|---|
| 50 mg | 5 mL |
| 150 mg | 15 mL |
| 450 mg | 45 mL |
| 600 mg | 60 mL |
Carboplatin Injection can be further diluted to concentrations as low as 0.5 mg/mL with 5% Dextrose in Water (D5W) or 0.9% Sodium Chloride Injection, USP.
When further diluted, Carboplatin Injection solutions are stable for 8 hours at room temperature (25°C). Since no antibacterial preservative is contained in the formulation, it is recommended that Carboplatin Injection solutions be discarded 8 hours after dilution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
HOW SUPPLIED
Carboplatin Injection 10 mg/mL sterile solution is available in the following presentations:
| 50 mg/5 mL vials, individually cartoned. (Blue flip-off seals) |
| 150 mg/15 mL vials, individually cartoned. (Blue flip-off seals) |
| 450 mg/45 mL vials, individually cartoned. (Blue flip-off seals) |
| 600 mg/60 mL vials, individually cartoned. (Blue flip-off seals) |
Unopened vials of Carboplatin Injection are stable for the life indicated on the package when stored at 25°C (77°F) [excursions permitted to 15°- 30°C (59°- 86°F) [see USP Controlled Room Temperature] and protected from light.
Carboplatin injection multidose vials maintain microbial, chemical, and physical stability for up to 15 days at 25°C following multiple needle entries.
HANDLING AND DISPOSALProcedures for proper handling and disposal of anti-cancer drugs should be considered. Several guidelines on this subject have been published1-7. There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
REFERENCES
- Recommendations for the Safe Handling of Parenteral Antineoplastic Drugs. NIH Publication No. 83-2621. For sale by the Superintendent of Documents, US Government Printing Office, Washington, DC 20402.
- AMA Council Report. Guidelines for Handling Parenteral Antineoplastics. JAMA 1985; 253(11):1590-1592.
- National Study Commission on Cytotoxic Exposure - Recommendations for Handling Cytotoxic Agents. Available from Louis P. Jeffrey, Sc.D., Chairman, National Study Commission on Cytotoxic Exposure, Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, Massachusetts 02115.
- Clinical Oncological Society of Australia. Guidelines and Recommendations for Safe Handling of Antineoplastic Agents. Med J Australia1983; 1:426-428.
- Jones RB, et al: Safe Handling of Chemotherapeutic Agents: A Report From the Mount Sinai Medical Center. CA-A Cancer Journal for Clinicians 1983; (Sept/Oct) 258-263.
- American Society of Hospital Pharmacists Technical Assistance Bulletin on Handling Cytotoxic and Hazardous Drugs. Am J Hosp Pharm 1990; 47:1033-1049.
- Controlling Occupational Exposure to Hazardous Drugs. (OSHA WORK-PRACTICE GUIDELINES). Am J Health-Syst Pharm 1995; 52:1669-1685.
Product Glimpse
Description
We are committed to work towards a healthier and happier world. The company is an integrated, research based international pharmaceutical company, producing a wide range of quality, affordable generic (Carboplatin 10mg/50mg Injection) medicines, trusted by health-care professionals and patients across geographies.
We offer you the highest quality new Generic medicines ie. Carboplatin 10mg/50mg Injection, drugs and also with innovative packing at the lowest prices shipped to you from India. Browse our latest Pharmaceuticals and Generics possibilities and other pharmaceuticals possibilities…more.